Novel Coinage metal complexes with sulfur
- from the gasphase to solid state chemistry
The concept of ”pseudo-gas-phase conditions” in the solid state allowed us to stabilize the first examples of undistorted homoleptic metal – S8 complexes, that is, the almost C4v - symmetric Ag(η4-S8)+ and the approximately centrosymmetric [Ag(η4-S8)2]+ cation.[1]
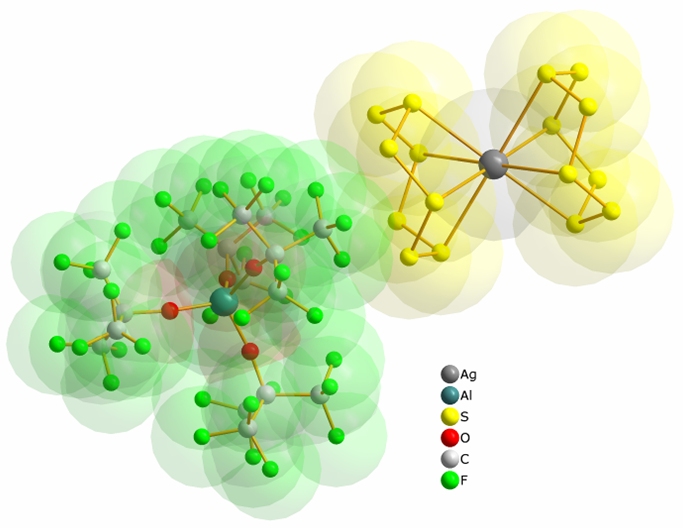
[Ag(S8)2][Al(OC(CF3)3)4]
The novel Cu(I)–sulfur complexes are the first examples of copper–cyclosulfur materials, and [Cu(S12)(S8)]+ and [Cu(CH2Cl2)(S12)]+ are the first examples of any metal with an S12 ring as a ligand. Furthermore, [Cu(S12)(S8)]+ is the first example of a complex with an element in two allotropic modifications as a ligand. The formation of the S12 complexes is induced by the pseudo gas-phase conditions generated by our best WCA ([Al(OC(CF3)3)4]).[2]
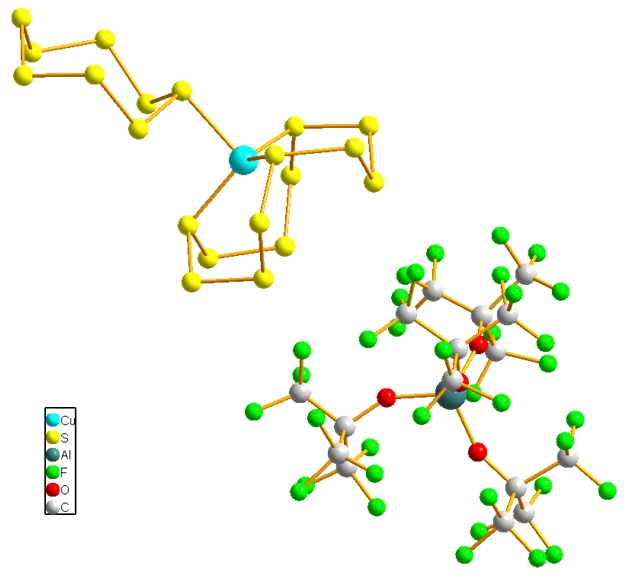
[Cu(S12)(S8)][Al(OC(CF3)3)4]
[1] T. S. Cameron, A. Decken, I. Dionne, M. Fang, I. Krossing, J. Passmore, Chem. Eur. J.2002, 15, 3386-3401.
[2] G. Santiso-Quinones, A. Higelin, J. Schaefer, R. Brückner, C. Knapp, I. Krossing, Chem. Eur. J. 2009, 6663-6677.

