Ga(I) and In(I) Salts
J. M. Slattery, A. Higelin, T. Bayer, I. Krossing, Angewandte Chemie International Edition 2010, 49, 3228-3231.
J. M. Slattery, A. Higelin, T. Bayer, I. Krossing, Angewandte Chemie 2010, 122, 3297-3301.
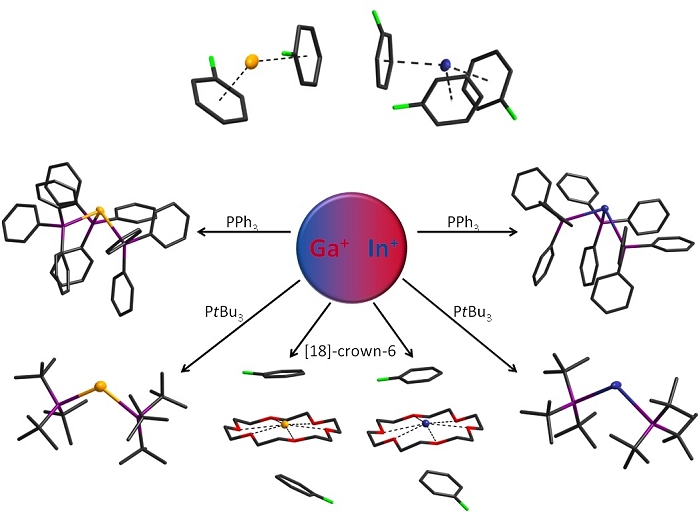
Elemental Gallium and Indium are oxidized to their monovalent salts by Ag[Al(OC(CF3)3)4] in aromatic solvents. This convenient synthesis yields arene supported Ga+ and In+ cations in multi-gramm scale which are highly available for further reactions. They represent an ideal starting material for the investigation of sub-valent group 13 elements.

These starting materials have in subsequent reactions led to previously unknown In(I) coordination complexes and the first ever examples of coordination chemistry at Ga(I). Crystallization from solutions of the M(I) salts with phosphanes and cyclic ethers yielded air sensitive crystals from colored solutions.
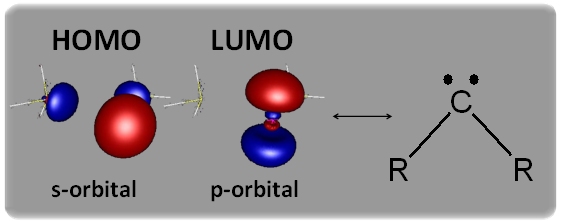
The tris-phosphane complexes show almost ideal trigonal pyramidal coordination, due to the donation of electron density from the ligands into the empty p-orbitals of the metal. The remaining lone-pair of the metal is found in the s-orbital shaped HOMO. Sterically more demanding phosphanes produce bis-complexes which show peculiar analogies to singulett carbenes. The HOMO is once again the s-orbital, while the LUMO is an empty p-orbital at the metal center.
The cyclic ether [18]-crown-6 forms planar complexes with the sub-valent metal cations. There is no interaction with the corresponding anion, only the coordination of two solvent molecules above and below the ring, which float just within the van-der-Waals range.