Research Overview
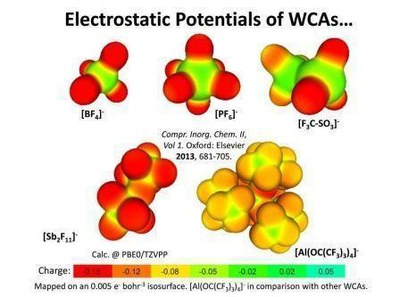
Weakly coordinating anions (WCAs)
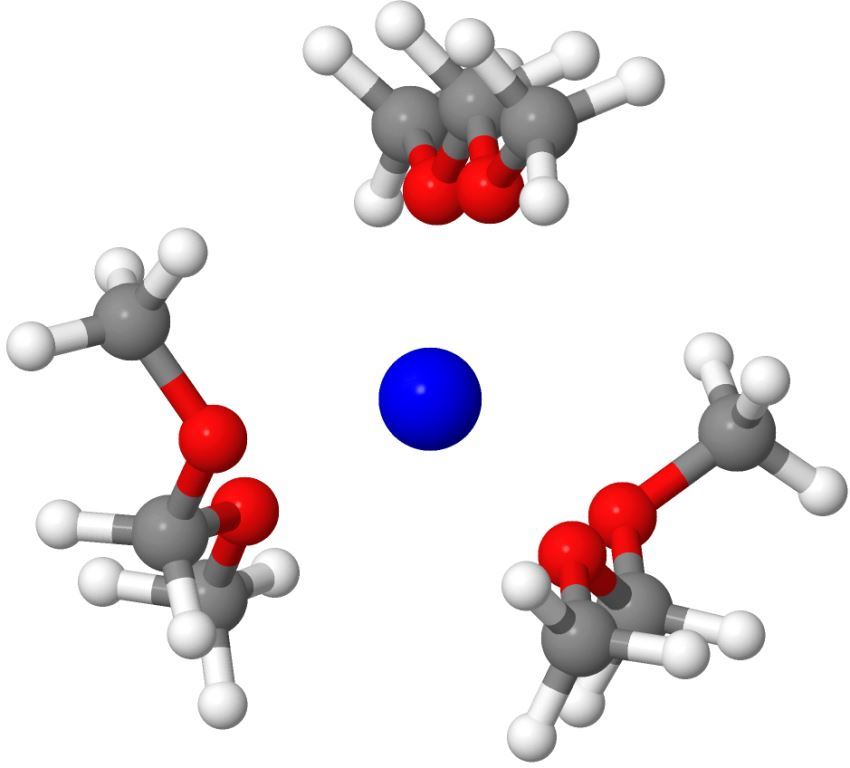
Fluorinated alkoxyaluminates [Al(ORF)4]– are WCAs that made their way over the past decades from a means to stabilize unusual cationic systems of fundamental interest to a well-developed class of materials that help to prepare problem case model compounds similarly to being useful in applied research, e.g. for Ionic Liquids (ILs), catalysis, polymerizations, electrochemistry and electrolytes. Currently we are aware of at least 50 groups worldwide that use the favorable properties of this chemically robust and easily in large scale or even commercially at www.iolitec.de available WCA class.
For more detailed information, please click here.
Key Publication: Chem. Eur. J. 2001, 7, 490.
Absolute Brønsted acities and redox potentials
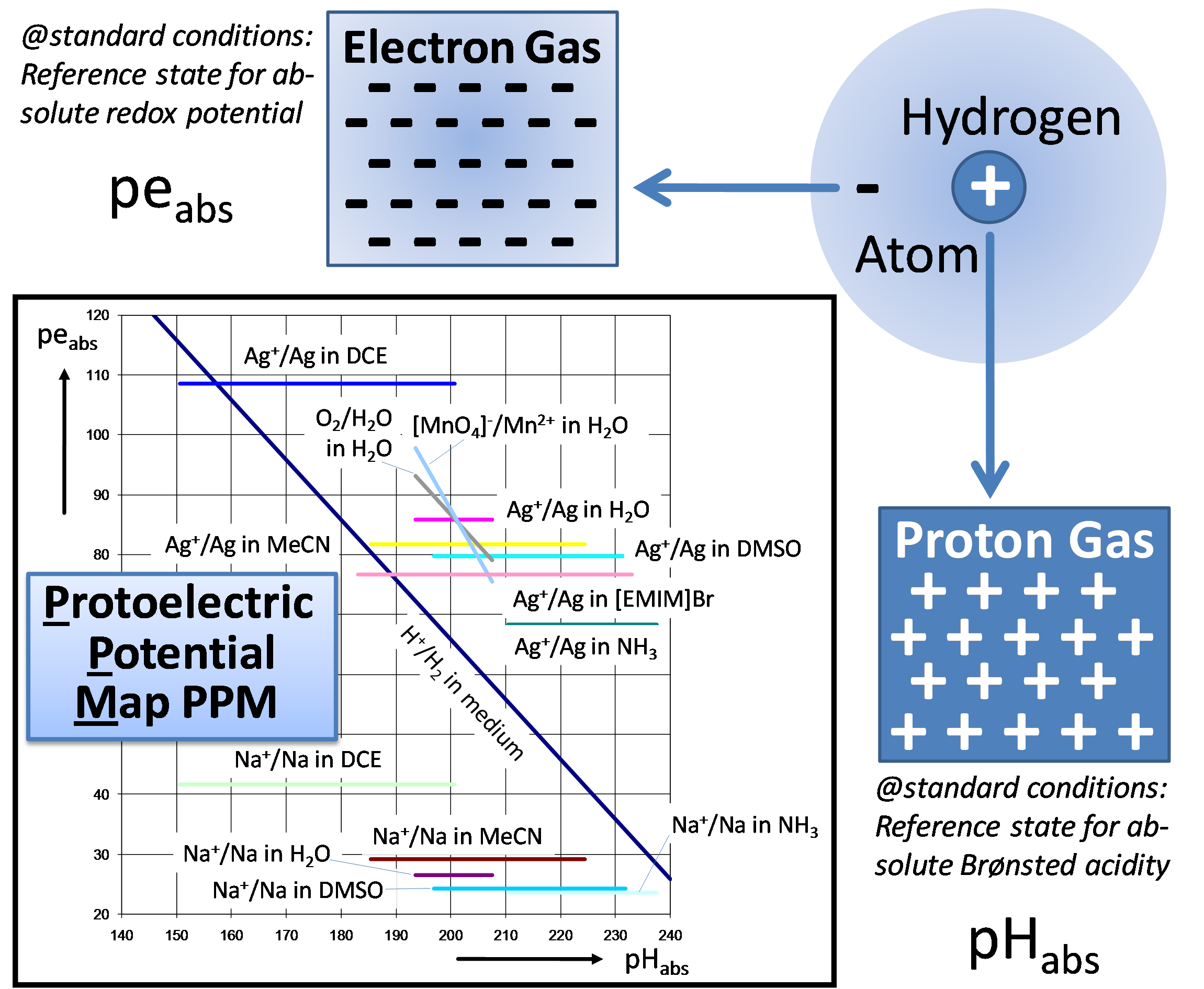
Although both acidities and redox potential are actually well understood, still no general possibility exists to compare these parameters in completely different environments, for instance water and benzene.
Spnsored by a grant of the European Research Council (ERC), we are tackling this problem with a mixture of sophisticated quantum-chemical calculations and (electro)chemical measurements. Through our work, already a unified pH scale, which is independent of solvents, could be estabilshed. A next step was the sp-called protoelectric potential map (PPM), which directly links acidities and redox potentials by means of fundamental thermodynamic laws.
For more detailed information, please click here.
Key publications: a) Chem. Eur. J. 2012, 18, 9333; b) Angew. Chem., Int. Ed. Engl. 2010, 49, 6885; c) Chem. Eur. J. 2014, 20, 4194.
Stabilization of reactive cations
Weakly coordinating anions enable the synthesis of highly reactive and exotic cations in condensed phase. The silver cation of the Ag+[Al(ORF)4]– and [(FRO)3Al-F-Al(ORF)3]– salts, for instance, is so polarizing that it abstracts halide from neutral CX4 and allows to form the series of simple CX3+ salts (X = Cl, Br, I).
With the superacidic system HBr/AlBr3, we could even stabilize the 2-norbornyl cation and solve its crystal structure for the first time, proving its carbonium character.
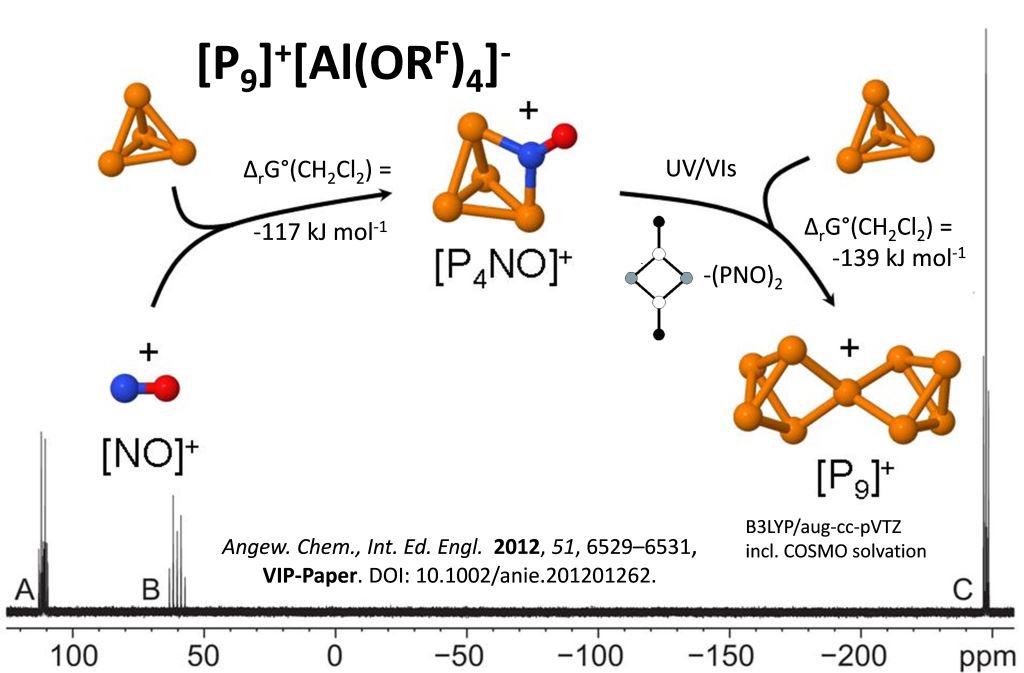
Six modifications of elemental phosphorus are known, and hundreds of structures of anionic homoatomic polyphosphides. But only in 2012 we reported the first homoatomic phosphorus cation in condensed phases, [P9]+.
For more detailed information, please click here.
Key publications: a) Science 2013, 341, 62. b) Angew. Chem., Int. Ed. Engl. 2012, 51, 6529, VIP-Paper. c) Dalton Trans. 2011, 1448.
Organometallic and subvalent complexes
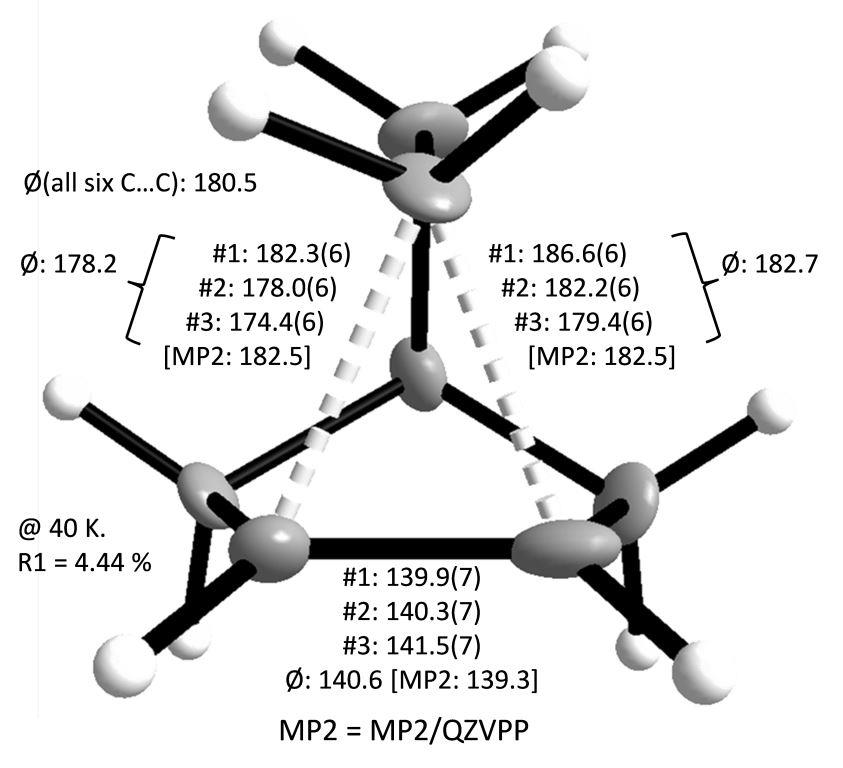
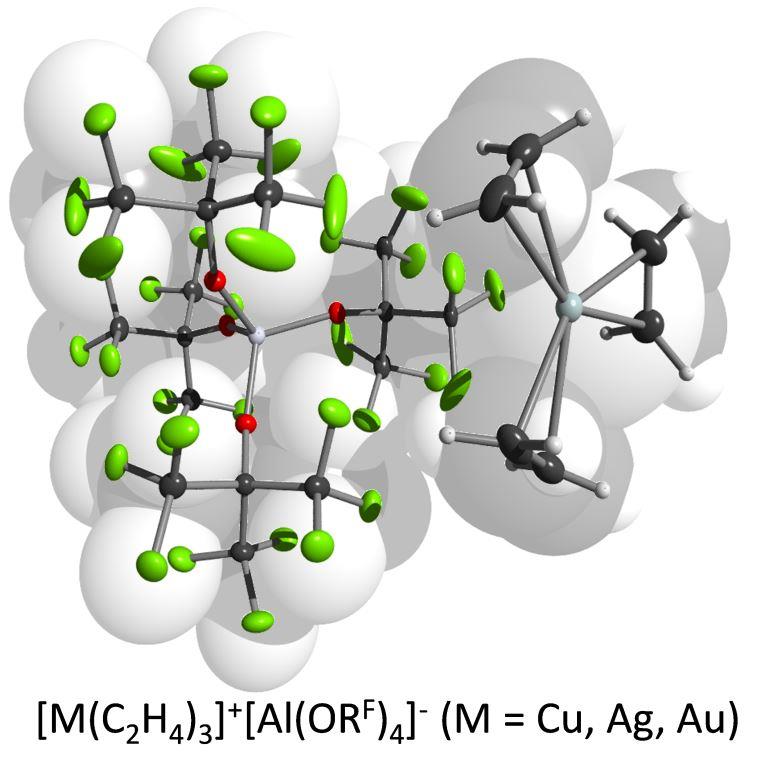
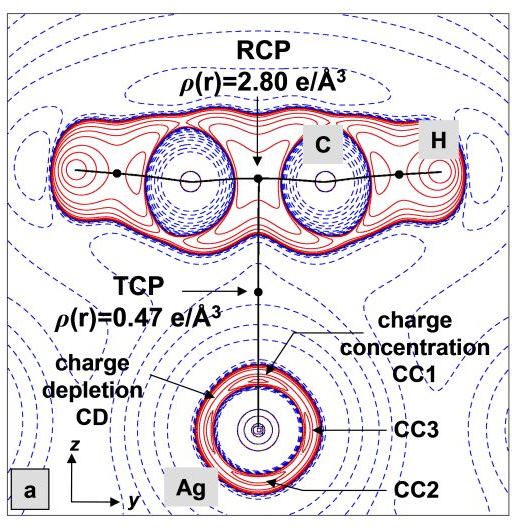
Many organometallic complexes can be synthesized with the help of highly fluorinated WCAs. Even ethylen, the simplest of all olefins, can be coordinated to each of the three coinage metal M+ ions. And with ethine as a ligand, silver even forms a [Ag(C2H2)4]+ complex, for which the first experimentally determined T-shaped charge density was established.
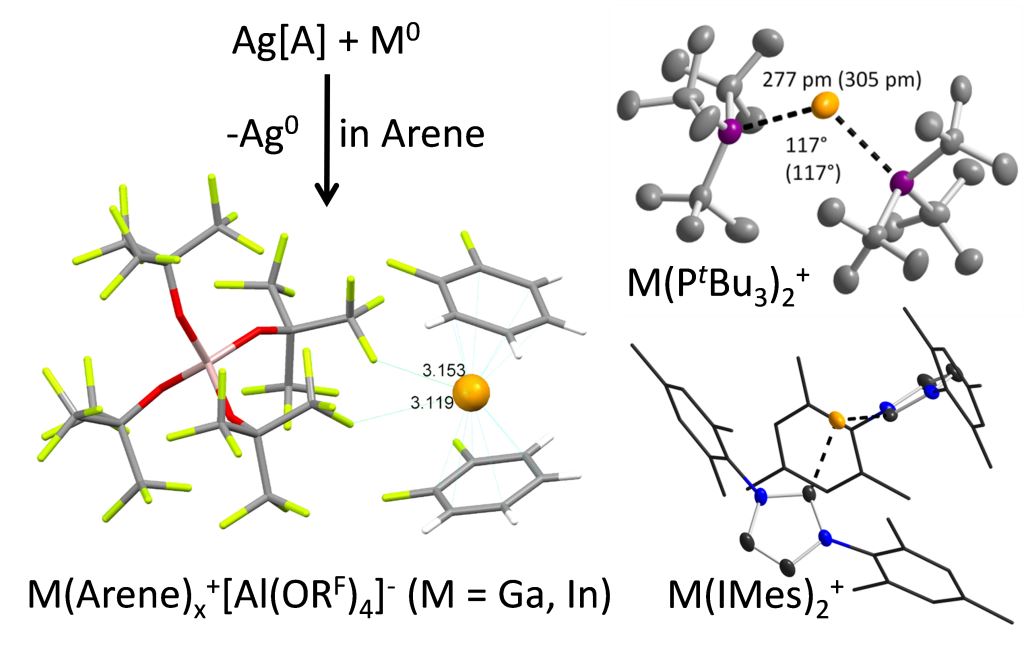
The heavier group 13 elements can selectively be oxidized with Ag+[Al(ORF)4]– in order to form the respective univalent M+ salts. In contrast to all other known univalent M+ (M = Ga, In) sources, this starting materials allowed to study the coordination chemistry of M+ with classical ligands like phosphines, ethers or carbenes.
For more detailed information, please click here.
Key publications: a) Angew. Chem. 2003, 115, 5907. b) Angew .Chem. 2007, 119, 8445. c) Angew. Chem. 2008, 120, 7914. d) Angew. Chem. 2010, 122, 3297 (VIP-Paper).
Ionic Liquids
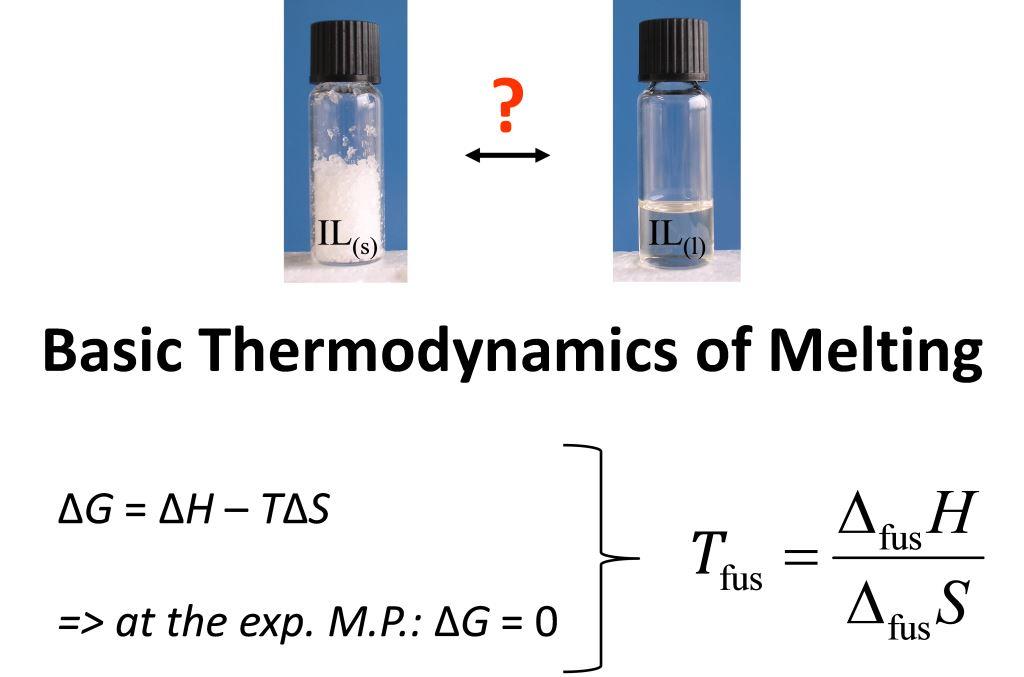
Ionic liquids (ILs) are salts with melting points below 100 °C and often even below room temperature. They offer a unique set of properties and thus are interesting systems both for fundamental and applied research.
One focus is the investigation of the thermodynamic principles, which govern the properties of ILs. We could show, for instance, that indeed entropy is the main factor for the (low) melting points of these salts. Our results can be used to predict many properties of ILs and eventually were implemented within the ILprop module of the software package CosmoThermX.
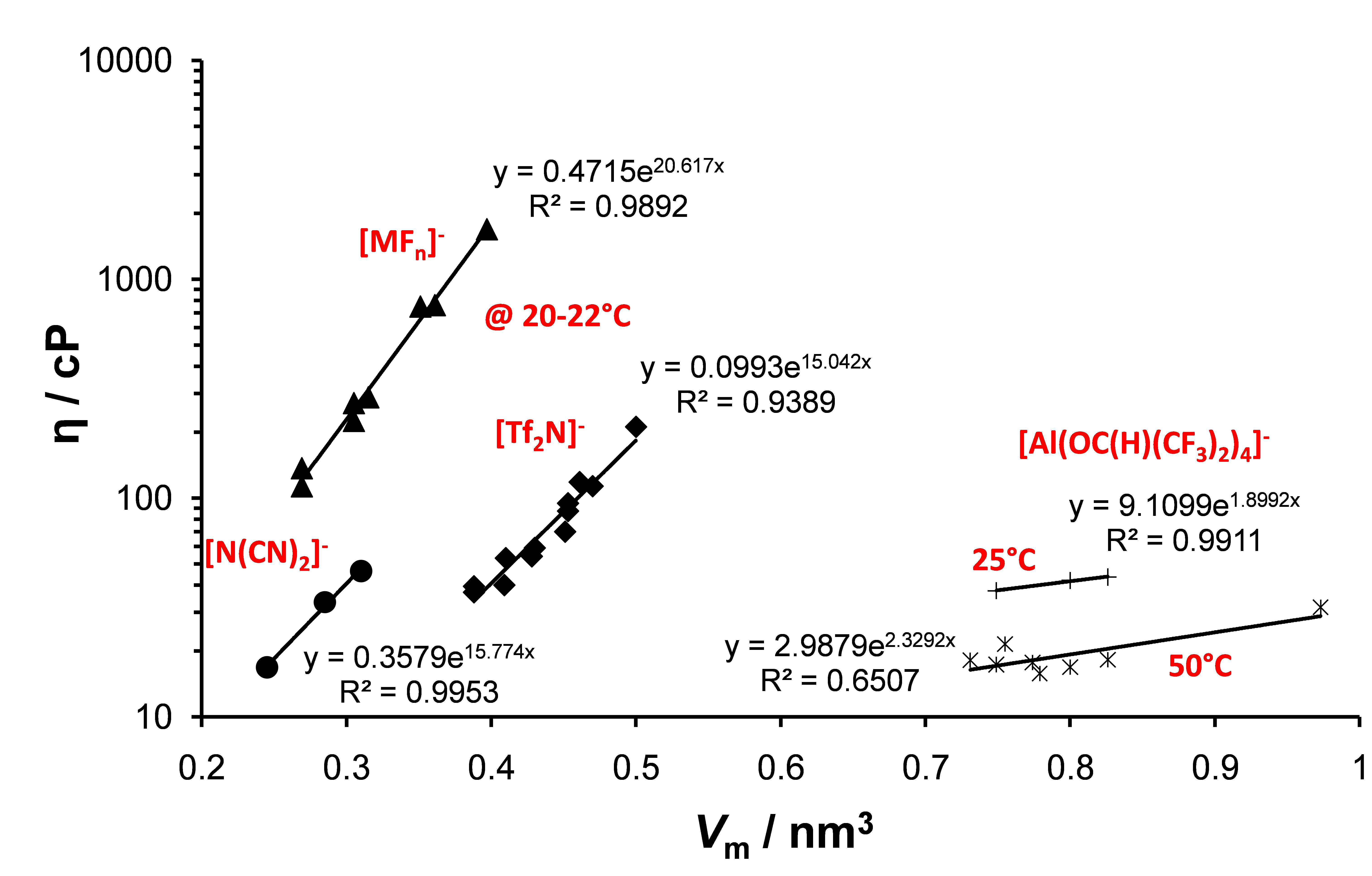
Another interest is the synthesis of new classes of ILs and the investigation of their physico-chemical properties, such as viscosity or electrical conductivity.
For more detailed information, please click here.
Key publications: a) J. Am. Chem. Soc. 2006, 128, 13427; b) J. Am. Chem. Soc. 2007, 129, 11296; c) Chem. Eur. J. 2011, 17, 6508; d) ChemPhysChem 2011, 12, 2959.
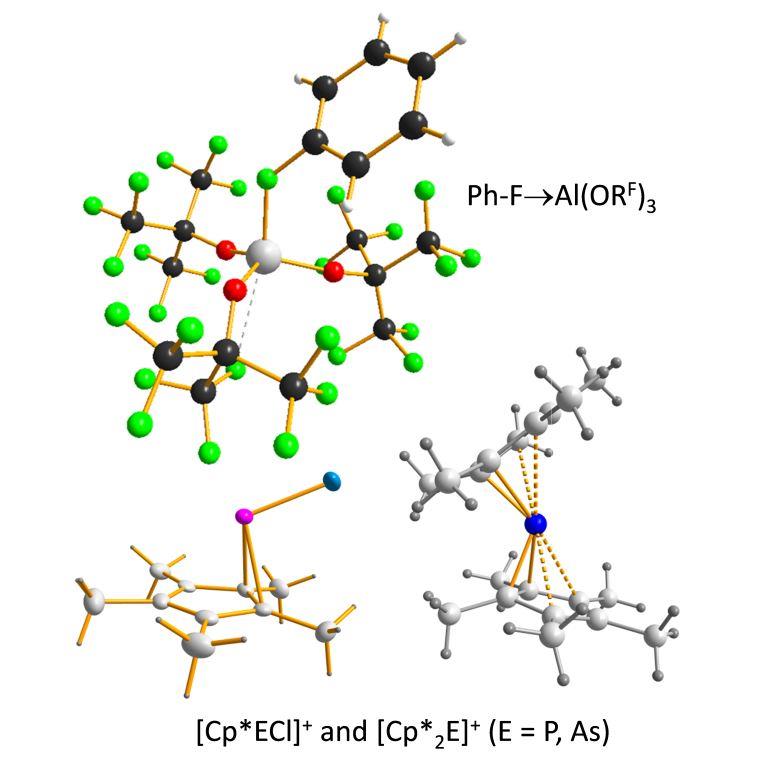
Lewis and Brønsted superacids
The Lewis acids underlying the fluorinated alkoxy-aluminates, i.e. Al(ORF)3, are very strong. In experimental work we have shown that they are stronger than the strongest conventionally applied Lewis acid SbF5 and have defined Lewis Superacidity as Lewis acids that are stronger than SbF5.
Al(ORF)3 coordinates very weak bases such as fluorobenzene PhF, SO2, FSiMe3 etc. and ionizes e.g. Cp*2ECl or Cp*ECl2 to give [Cp*2E]+ or [Cp*ECl]+ (E = P, As).
For more detailed information, please click here and here.
Key publications: a) Angew. Chem. Int. Ed. Engl. 2008, 47, 7659; b) Chem. Eur. J. 2011, 17, 12975; c) Chem. Eur. J. 2013, 20, 1218.
Energy storage
Several projects in our group are concerned with the development and improvement of energy storage technologies, from batteries to supercapacitors and even redox-flow batteries. A great share of this research is funded by a collaboration with BASF SE.
For more detailed information, please click here.
Key publications: a) ChemPhysChem 2015, 16, 666; b) Use of Lithium Alkoxyborates and Lithium Alkoxyaluminates as Conducting Salts in Electrolytes of Lithium Sulphur Batteries, WO/2015/007586, issued 19.07.2013.

Energy conversion
The conversion of chemical energy into, for instance, electrical energy or heat (and vice versa) is of great importance for our society. However, most energy conversion systems widely used today are accompanied by environmental issues such as pollution or global warming, above all the combustion of fossil fuels and the release of CO2 into the atmosphere.
A possible way to actually use CO2 as energy storage medium, or as "C1 starting material" for basic chemicals, is its conversion into methanol. Therefore, we are investigating heterogeneous catalysts for the conversion of CO2 into methanol with H2. Our goal is to find catalysts which allow a high product yield at relatively low temperatures and pressure. For this purpose, a variety of analysis methods is present, from BET and EDX systems to complete test rigs.
For more information, please click here.
Key publications: a) ACS Appl Mat Int 2014, 6, 1576-1582; b) ChemCatChem 2014, 6, 1721-1730.

Computer Chemistry
Almost every project in our group is accompanied by in silico calculations. Applications of computational methods range from the calculation of reaction energies and vibrational spectra to the prediction of solubilities or melting points. Hence, computational methods are an integral part of our work, and enable us to better understand the chemical systems which we investigate.
For more information, please click here.
Key publications: a) Chem Eur J 2011, 17, 5808-5826; b) J. Phys. Chem. B 2010, 114, 11133–11140.