Superweak Complexes of P4 with Ag(I) of WCAs
Superweak Complexes of Tetrahedral P4 Molecules with the Silver Cation of Weakly Coordinating Anions
The silver aluminates Ag[Al(OC(CF3)2(R))4] (R = H, CH3, CF3) react with solutions of white phosphorus P4 to give complexes that bind one or two almost undistorted tetrahedral P4 molecules in η2 fashion:
[Ag(P4)2][Al(OC(CF3)3)4] (1) containing the first homoleptic metal- posphorous cation, the molecular species (P4)Ag[Al(OC(CH3)(CF3)2)4] (2), and the dimeric Ag(μη2-P4)Ag bridged ((P4)Ag[Al(OC(H)(CF3)2)4])2 (3).[1]
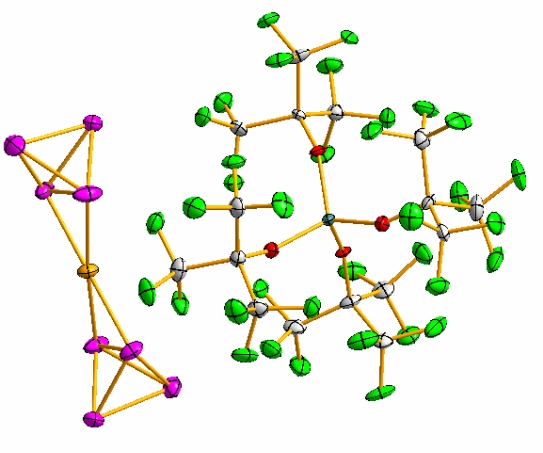
[M(P4)2][Al(OC(CF3)3)4]
M = Ag, Cu
To a first approximation the Ag atom in Ag(P4)2 is linearly coordinated by the two electron pairs of the two coordinated PP bonds to give a mainly electrostatic complex. The final small preference for the planar coordination environment of the Ag atom is mediated by an unusual backbonding [dx2-y2(Ag)*(PP)] from the closed 4d10 shell of silver, which minimizes the unfavorable positive charge on the phosphorus atoms. This shows that nonclassical and weak bonding modes in weak cationic Lewis acid ± base complexes can be stabilized by minimizing the electrostatic cation ± anion interaction between the species.[1] Furthermore, the analog copper complex is stabilized in our group too.
[1] I. Krossing, L. van Wüllen, Chem. Eur. J. 2002, 8, No .3, 700-711

