Ionic Liquids
 | General Synthesis of Ionic Liquids Ionic Liquids based on the Weakly Coordinating [MIII(ORF)4]- Anions (M = Al or B, RF = C(H)(CF3)2) Synthesis of [Al(hfip)4]- ILs (hfip: hexafluoroisopropoxy, "OC(H)(CF3)2") Synthesis of the WCA salt Na+[B(hfip)4]- and its application to prepare ILs Synthesis and Characterization of Na+[B(hfip)4]-.(Solvent)x Synthesis and Characterization of Ionic Liquids with the [B(hfip)4]- Anion Physical Data of other ILs - Own Determinations |
Introduction
Ionic Liquids (ILs) are an expanding and one of the most popular material-classes within the last 15-20 years. Due to their unusual properties, such as extremely low vapor pressures, interesting transport as well as electrochemical properties, they attracted much recent attention. The increasing interest to use ILs in industrial applications accelerated the IL-research rapidly and the importance to understand their nature and construction rose consequently. Their characterizing features are the liquid state and non-molecular, ionic character at low temperatures. The ILs differ clearly from molten salts, which are known in general as high-melting, -viscous and corrosive media. Differently, the ILs are liquid at relatively low temperatures and consequently they are more attractive and friendlier in processing. The boiling temperature of water is usually accepted as the melting-limit of ILs.
ILs are generally defined as salts composed solely of ions with melting temperatures below 100°C.
However, the most interest in IL-chemistry lies in "room temperature ionic liquids" (RTILs), which are liquid even at r.t. Even if in some cases molten salts were used successfully as reaction media in high temperature-syntheses, the replacement of conventional, organic solvents usually requires the liquid state below 100°C.
What is the difference between a conventional salt as NaCl and an IL? Why does the first melt at about 800 °C while the second melts below 100 °C? These questions are probably the first characteristic ones, if one wants to compare conventional salts and ILs. The difference lies certainly in the anion-cation interactions, which are strong for small ions with a poor charge distribution and weak for larger ones with a good charge distribution. Probably due to the negative charge, the anion is of utmost importance for the interactions and plays a determining role for both the melting process and other fundamental physical properties, e.g. viscosity, conductivity or density. In our group, we deal with such type of anions and their special features in relation to weak coordination, an ability which make them unrenounceable for ILs and many other applications (see Weakly Coordinating Anions).
The most important ingredient of ILs is surely the low cation-anion interactions. This is the reason for two most important features of ILs; firstly it is responsible for the melting process at low temperatures, and secondly it is the basis of their ionic construction. By increasing of cation-anion interactions, an IL passes over either to a higher melting salt or to a molecular liquid. Thus, the decrease of ionic interactions is the leading factor to obtain ILs with optimized properties, which is only be possible by using suitable ions. In Scheme 1, some of the widely used anions and cations in modern IL-chemistry are shown.
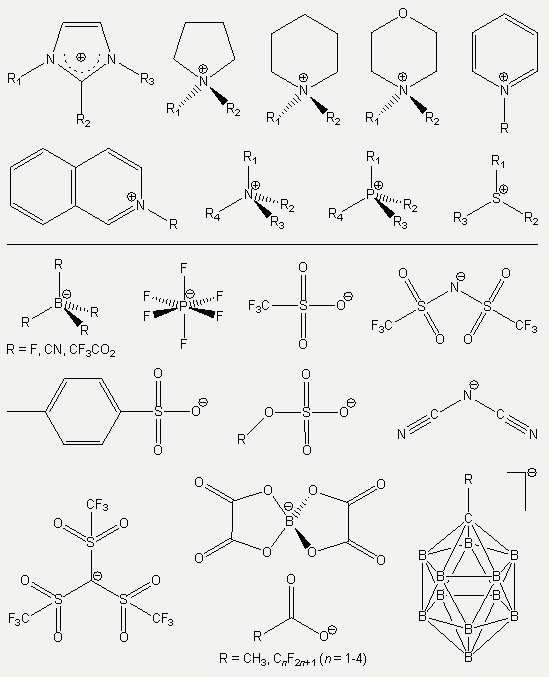
Scheme 1: Some examples for widely used IL ions.
More Details
General Synthesis of Ionic Liquids
Ionic Liquids based on the Weakly Coordinating [MIII(ORF)4]- Anions (M = Al or B, RF = C(H)(CF3)2)
Synthesis of [Al(hfip)4]- ILs (hfip: hexafluoroisopropoxy, "OC(H)(CF3)2")
Synthesis of the WCA salt Na+[B(hfip)4]- and its application to prepare ILs
Synthesis and Characterization of Na+[B(hfip)4]-.(Solvent)x
Synthesis and Characterization of Ionic Liquids with the [B(hfip)4]- Anion
Physical Data of other ILs - Own Determinations

