ILs with [B(hfip)4]- Anion
Synthesis and Characterization of Ionic Liquids with the [B(hfip)4]– Anion
Through anion metathesis in dry Et2O with stirring at r.t. for 24 h as shown in Scheme 4, the sodium cation of the clean mono-DME adduct was exchanged for substituted imidazolium and morpholinium cations. Then the mixture was filtered over a G4 Schlenk frit giving clear filtrates that did not show any indication of AgX formation upon addition of aqueous AgNO3 solution.
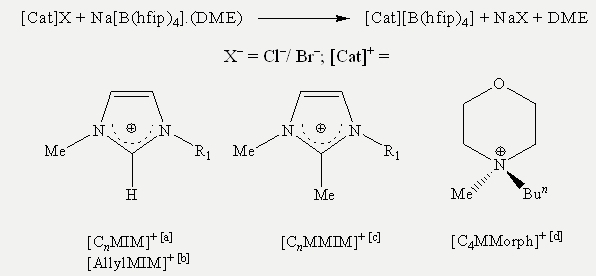
Scheme 4: Cations used: [a] [CnMIM]+ = 1-alkyl-3-methylimidazolium, where n is the length of the alkyl chain.
[b] [AllylMIM]+ = 1-allyl-3-methylimidazolium. [c] [CnMMIM]+ = 1-alkyl-2,3-dimethylimidazolium, where n is the
length of the alkyl chain. [d] [C4MMorph]+ = N-butyl-N-methylmorpholinium.
From these filtrates, the solvent was removed by vacuum distillation and the IL-salts were dried in vacuum (0.1 Pa) until no traces of DME were visible in the 1H-NMR spectra (5 to 24 h). Typical yields of the purified product were 93 to 98%.
Table 2: Melting points Tm, crystallization temperatures Tc and decomposition temperatures Td of the [B(hfip)4]– salts (average values of two or three determinations).[18]
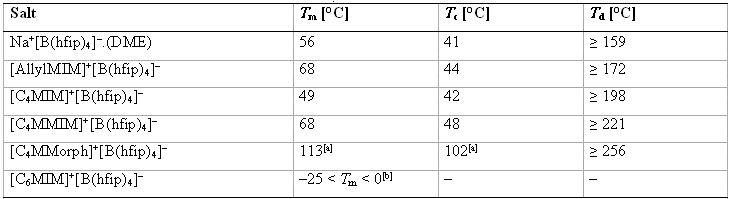 [a] The DSC investigation showed two endothermic signals during heating (at 78 and 113°C) and two exothermic signals during cooling (at 63 and 102°C). The melting and crystallization temperatures from the DSC analysis were confirmed by measuring the sample manually. Additional signals, the endothermic (78°C) and the exothermic one belong to the first event (63°C), indicate a phase transition or polymorphism of the salt.
[a] The DSC investigation showed two endothermic signals during heating (at 78 and 113°C) and two exothermic signals during cooling (at 63 and 102°C). The melting and crystallization temperatures from the DSC analysis were confirmed by measuring the sample manually. Additional signals, the endothermic (78°C) and the exothermic one belong to the first event (63°C), indicate a phase transition or polymorphism of the salt.
[b] Observed manually by using refrigerators with the indicated cooling temperatures.
Stability against Humidity and Water: To test the [B(hfip)4]– IL stability against humidity and water, the [C4MIM]+[B(hfip)4]– IL was put into an open beaker and left in air for 1 d, after which a NMR sample was prepared in CD2Cl2 and measured. After this measurement, one drop of water was added intentionally and after intense shaking the fate of the sample was monitored over time by NMR. It should be noted that CD2Cl2 is not very miscible with water and thus the contact of both phases in the NMR tube may have been limited.
Possibly, the [B(hfip)4]– anion decomposes very slowly with formation of boric acid and hfipH. Since boric acid is insoluble in CD2Cl2, in solution only the remaining [B(hfip)4]– IL and the upon hydrolysis formed alcohol (hfipH) should be visible in the 1H- and 19F-NMR investigations. NMR spectra show that after leaving the sample in the air, immediately after adding water as well as 8 h after the addition of water no trace of decomposition of [C4MIM]+[B(hfip)4]– was observed. After 5 d a slight clouding (possibly hydrolysis product boric acid) was observed in the NMR tube, which was accompanied by the observation of small traces of hfipH (ca. 1% by NMR). These investigations suggest that the [B(hfip)4]– ILs may be safely handled in air, but not extracted for prolonged times with water.
Literature
[18] S. Bulut, P. Klose, I. Krossing, Dalton Trans. 2011, 40, 8114

