Synthesis of [Al(hfip)4]– ILs
Ionic Liquids based on the Weakly Coordinating [MIII(ORF)4]– Anions (M = Al or B, RF = C(H)(CF3)2)
The physical properties of an IL are connected to many physical observables, where the anion-cation interactions and the size of the ions as measured for example by the molecular volume probably play the most important role. The strength of these anion-cation interactions are correlated to some extent with the coordination ability and Lewis basicity of the anion. Thus, weakly coordinating anions (WCA) are good candidates for the synthesis of new salts with lower melting points and other optimized physical properties.
Synthesis of [Al(hfip)4]– ILs (hfip: hexafluoroisopropoxy, “OC(H)(CF3)2”)[12]
In our group [Al(ORF)4]– WCAs with fluorinated alkoxy residues ORF = OC(CF3)3, OC(CH3)(CF3)2 or OC(H)(CF3)2 are used since 1999 to successfully stabilize reactive and other cations. But the [Al(hfip)4]– aluminate forms ― unexpectedly for the large size of the anion ― also RTILs using suitable cations with very interesting physical properties such as low viscosity, high conductivity, high dielectric constant, very good ionicity, very good hydrogen solubility and high electrochemical stability (> 5.0 V vs. Li/Li+). Since the polyfluoroalkoxyaluminates have a lower Lewis basicity and coordinating ability than conventional solvents, the use of [Al(hfip)4]– ILs appears to be very interesting as reaction media. ILs with [Al(hfip)4]– as the anion are thus of interest due to the high electrochemical stability of this anion as well as for the stabilization of highly reactive, redox-active cations.
In the first step, Li[Al(hfip)4] was synthesized by reacting pure Li[AlH4] and dry hexafluoroisopropanol in dry hexane.
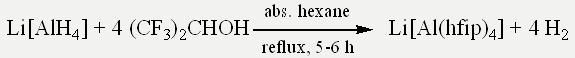
The reaction works very well with yields exceeding 95% for the optimized procedure. In the anion metathesis, lithium was exchanged for substituted ammonium, imidazolium, pyrrolidinium, pyridinium, piperidinium and morpholinium cations (by using halide salts of the cations, X– = Cl–/ Br–).
![]()
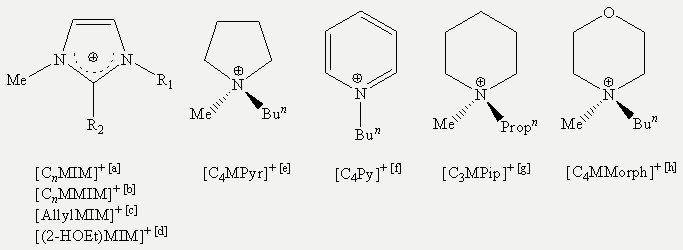
Scheme 3: Cations used in the reaction shown above: [a] [CnMIM]+ = 1-alkyl-3-methylimidazolium, where n is the length of the alkyl chain. [b] [CnMMIM]+ = 1-alkyl-2,3-dimethylimidazolium, where n is the length of the alkyl chain. [c] [AllylMIM]+ = 1-allyl-3-methylimidazolium. [d] [(2-HOEt)MIM]+ = 1-(2-hydroxyethyl)-3-methylimidazolium. [e] [C4MPyr]+ = N-butyl-N-methylpyrrolidinium. [f] [C4Py]+ = N-butylpyridinium. [g] [C3MPip]+ = N-propyl-N-methylpiperidinium. [h] [C4MMorph]+ = N-butyl-N-methylmorpholinium.
Anion metathesis took place in abs. CH2Cl2; after stirring at r.t. for 24 h the mixture was filtered over siliceous earth (which was the best of all tested filter materials to eliminate the LiCl precipitate). After filtration, the solvent was removed by vacuum distillation. The remaining product was rinsed with abs. hexane and dried in vacuum (0.1 Pa). Typical yields of the purified product are 90 to 95%.
Table 1: Melting points Tm and crystallization temperatures Tc of the [Al(hfip)4]– ILs.[6, 12]
[a] No crystallization peak was observed in the working temperature ranges (measurements were carried out starting from RT for these ILs).
Thermal Stability: The thermal decomposition of the symmetrical ammonium salts of the [Al(hfip)4]– anion started above 150°C. Other ILs were heated to 150–250 °C during DSC investigations. From the DSC-traces no clear single decomposition pathway could be recognized. However, clear decomposition signals for [C2MMIM]+[Al(hfip)4]–, [C4MPyr]+[Al(hfip)4]– or [C4MMorph]+[Al(hfip)4]– were observed from 172, 155 and 164°C, respectively. From these measurements it follows that thermal decomposition of [Al(hfip)4]– based ILs with different types of the cations begins around 150°C.
Literature
[6] I. Raabe, K. Wagner, K. Guttsche, M. Wang, M. Grätzel, G. Santiso-Quiñones, I. Krossing, Chem. Eur. J. 2009, 15, 1966.
[12] S. Bulut, P. Klose, M.-M. Huang, H. Weingärtner, P. J. Dyson, G. Laurenczy, C. Friedrich, J. Menz, K. Kümmerer, I. Krossing, Chem. Eur. J. 2010, 16, 13139.