WCA salt Na+[B(hfip)4]-
Ionic Liquids based on the Weakly Coordinating [MIII(ORF)4]– Anions (M = Al or B, RF = C(H)(CF3)2)
The physical properties of an IL are connected to many physical observables, where the anion-cation interactions and the size of the ions as measured for example by the molecular volume probably play the most important role. The strength of these anion-cation interactions are correlated to some extent with the coordination ability and Lewis basicity of the anion. Thus, weakly coordinating anions (WCA) are good candidates for the synthesis of new salts with lower melting points and other optimized physical properties.
Synthesis of the WCA salt Na+[B(hfip)4]- and its application to prepare ILs [18]
Several borate based weakly coordinating anions (WCAs) are known, e.g. for stabilizing reactive cations, for IL synthesis or in catalyst as well as in electrochemical applications.
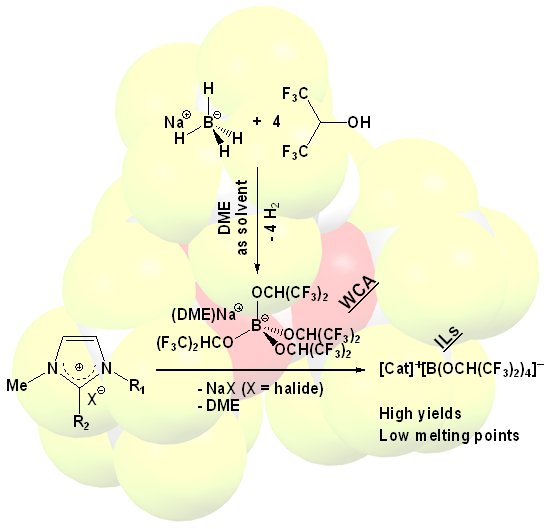
A disadvantage of all [Al(hfip)4]- salts shown above is their water sensitivity; decomposition of the anion is already observed in air due to humidity. We reasoned that a homologous anion with boron as central atom should be more stable against water, because B-O bonds are shorter and less polar than Al-O bonds. To the best of our knowledge, no ORF analogs (ORF as above) with boron as central atom were reported yet, probably due to the large steric impediment introduced by binding four large alkoxy groups to the small boron atom. In agreement with this, the attempted synthesis of Na[B(hfip)4] from NaBH4 and hfipH (hexafluoroisopropanol) was earlier shown to proceed only up to Na+[H-B(hfip)3]-, while [B(OR)4]- salts with smaller alkoxides like OCH3 or OCH2CF3 were straight forward to prepare.
Following we show a straight forward synthesis of the hitherto unknown Na[B(hfip)4] salt, as well as metathesis reactions leading to [B(hfip)4]- ILs.
Literature
[18] S. Bulut, P. Klose, I. Krossing, Dalton Trans. 2011, 40, 8114

