General Synthesis of Ionic Liquids
In general, the preparation of new materials is strongly coupled with the considerations about the purity of the ILs afterwards. This is more in focus, if it is desired to use those in applications, which dependent strongly on the physical properties and in turn on the purity of the compounds.
Generally, there are various methods to synthesize ILs, where the selection of the correct method depends on the type of aimed IL. More precisely, since most of the IL-cations are typically available as halide salts, the desired anion and its available precursor are mostly decisive for the selection of the suitable synthesis-method. The synthesis of ILs can roughly be separated in two routes (summarized in Scheme 2):
- The first one is a one-way reaction, in which desired ILs are produced directly from their starting materials. Hereby, the cation and anion are formed together in the same working step.
- The second one is a poly-way reaction, where desired ILs are synthesized at least via two reaction-steps, namely the synthesis of the cation as a salt with an easily changeable anion such as halides (quaternization, step 1) and the anion metathesis (step 2). As said, this synthetic route works at least by two reaction-steps, because according to the desired cation its preparation may take more than one step (e.g. highly substituted cations). Anion metathesis can be realized in various ways in accordance with the available anion source and preference of the method leading to minimal degree of impurities as possible.
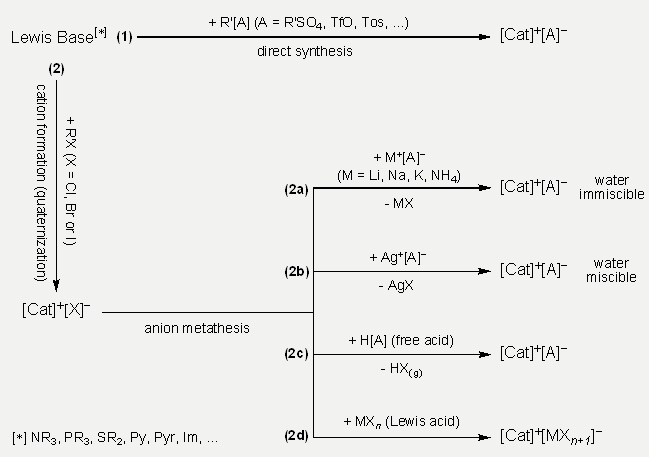
Scheme 2: General used synthesis routes for ILs (abbr.; Py: pyridine, Pyr: N-alkyl pyrrolidine, Im: N-alkyl imidazole, TfO: triflate and Tos: tosylate).
Group Publications in IL research:
- I. Krossing, J. M. Slattery, C. Daguenet, P. J. Dyson, A. Oleinikova, H. Weingärtner, J. Am. Chem. Soc. 2006, 128, 13427.
- C. Daguenet, P. J. Dyson, I. Krossing, A. Oleinikova, J. M. Slattery, C. Wakai, H. Weingärtner, J. Phys. Chem. B 2006, 110, 12682.
- I. Krossing, J. M. Slattery,Z. Phys. Chem. 2006, 220, 1343.
- H. Weingärtner, P. Sasisanker, C. Daguenet, P. J. Dyson, I. Krossing, J. M. Slattery, and T. Schubert, J. Phys. Chem. B 2007, 111, 4775.
- J. M. Slattery, C. Daguenet, P. J. Dyson, T. J. S. Schubert, I. Krossing, Angew. Chem. Int. Ed. 2007, 46, 5384.
- I. Raabe, K. Wagner, K. Guttsche, M. Wang, M. Grätzel, G. Santiso-Quiñones, I. Krossing, Chem. Eur. J. 2009, 15, 1966.
- U. Preiss, J. M. Slattery, I. Krossing, Ind. Eng. Chem. Res. 2009, 48, 2290.
- U. Preiss, C. Jungnickel, L. Thöming, I. Krossing, J. Luczak, M. Diedenhofen, A. Klamt, Chem. Eur. J. 2009, 15, 8880.
- P. Eiden, Q. Liu, S. Zein El Abedin, F. Endres, I. Krossing, Chem. Eur. J., 2009, 15, 3426.
- U. Preiss, S. Bulut, I. Krossing, J. Phys. Chem. B 2010, 114, 11133.
- Ulrich Preiss, V. N. Emel’yanenko, S. P. Verevkin, D. Himmel, Y. U. Paulechka, and I. Krossing, ChemPhysChem 2010, 11, 3425.
- S. Bulut, P. Klose, M.-M. Huang, H. Weingärtner, P. J. Dyson, G. Laurenczy, C. Friedrich, J. Menz, K. Kümmerer, I. Krossing, Chem. Eur. J. 2010, 16, 13139.
- M.-M. Huang, S. Bulut, I. Krossing, H. Weingärtner, J. Chem. Phys. 2010, 133, 101101.
- G. Dlubek, Yang Yu, R. Krause-Rehberg, W. Beichel, S. Bulut, N. Pogodina, I. Krossing, Ch. Friedrich, J. Chem. Phys. 2010, 133, 124502.
- P. Eiden, S. Bulut, T. Köchner, Ch. Friedrich, T. Schubert, I. Krossing, J. Phys. Chem. B 2010,115, 300.
- C.-W. Cho, U. Preiss, C. Jungnickel, S. Stolte, J. Arning, J. Ranke, A. Klamt, I. Krossing, J. Thöming, J. Phys. Chem. B 2011, 115, 6040.
- S. Bulut, P. Eiden, W. Beichel, J. M. Slattery, T. Beyersdorff, T. Schubert and I. Krossing, ChemPhysChem 2011, 12, 2296.
- S. Bulut, P. Klose, I. Krossing, Dalton Trans. 2011, 40, 8114.
- U. Preiss, S. P. Verevkin, T. Koslowski, and I. Krossing, Chem. Eur. J. 2011, 17, 6508.

