Na+[B(hfip)4]-.(Solvent)x
Synthesis and Characterization of Na+[B(hfip)4]–.(Solvent)x
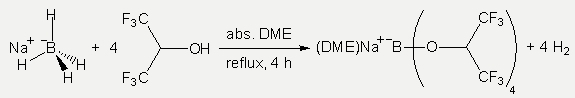
The synthesis of Na+[B(hfip)4]– was investigated by several reaction pathways out of which only a one pot reaction, starting from NaBH4 and hfipH, was successful.
By contrast to these difficulties, the synthesis of the related Li+[Al(ORF)4]– (RF = C(H)(CF3)2, C(CH3)(CF3)2 or C(CF3)3) works well using LiAlH4 and the fluorinated alcohol in refluxing hexane or heptane suspension. Two distinct differences between the synthesis of the aluminate and borate salts exist:
i) the basicity of the hydrides in [BH4]– is lower than that in [AlH4]–;
ii) boron is smaller than aluminum and thus a higher steric impediment can be expected when binding four large alkoxy groups to a central boron atom.
Both differences are negative for the synthesis of the Na+[B(hfip)4]– salt, and in agreement with earlier findings, incomplete conversions were observed in many cases. Usually the first three hydrides reacted fast and completely. But substituting the fourth hydride is difficult. Best results were finally obtained in 1,2-dimethoxyethane (DME), in which the conversion was complete within 4 h reflux. Depending on the solvent used, THF or DME solvates crystallize from the solutions. After complete conversion in THF (NMR-check), the solvent was removed by vacuum distillation and the product was dried in vacuum (0.1 Pa) at r.t. for 2 d. Single crystal X-ray analysis revealed the coordination of two THF molecules giving Na(THF)2+[B(hfip)4]–. When DME was used, the solvent was removed by vacuum distillation after completion of the reaction (NMR check), and the product was dried at r.t. in vacuum (0.1 Pa) for 3 d leading to a material consistent with the formulation Na(DME)3+[B(hfip)4]– (NMR). The DME content could be reduced to Na(DME)+[B(hfip)4]– by heating to 40–45 °C in the vacuum (0.1 Pa) for 10 h. All NMR investigations point to a clean synthesis in DME with no visible by- or decomposition-products. Na(DME)+[B(hfip)4]– turned out to be the preferred starting material for further reactions.

