Reactive Cations
 |
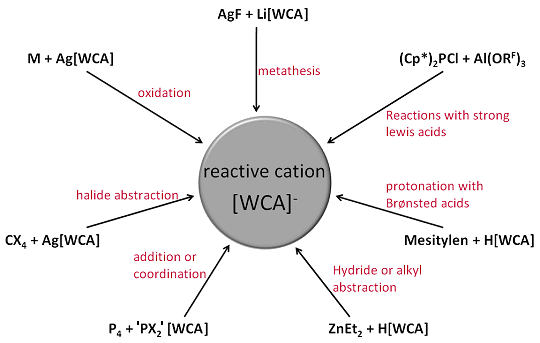
Metathesis reactions with other salts Reactions with strong Lewis acids Reactions with Brønsted acids Halide abstraction Hydride and Alkyl abstraction Addition or coordination to the cation of a WCA salt Direct oxidation of a substrate |
This reaction scheme contains interactive buttons
Stabilization of reactive cations with weakly coordinating anions
Weakly coordinating anions allow the stabilization of reactive cations. This is achieved through the chemical robustness (steric shielding and electronic deactivation of the most basic sites) of the anion as well as the minimization of the overall cation-anion interactions. Due to the large size of weakly coordinating anions ( > 1 nm) their salts have very low lattice energies. This is a direct effect of the large separation of cations and anions. The lattice energies may even be as low as the sublimation enthalpies of molecular solids of comparable atomic weight. Hence, the cations don’t feel strong electric fields as they would in classical salts. Their situation rather resembles that of a particle in the gas phase. The remaining dispersive interactions with the highly fluorinated surfaces of the anions are weak and hardly structure determining. We therefore frequently refer to pseudo gas phase conditions. In general, weakly bound cationic complexes are stabilized in WCA salts as there is little gain in lattice enthalpy upon decomposition of the complex.
Reactive cations in WCA salts can be generated from starting materials like Li[PF], Ag[PF], Al(ORF)3 or H(OEt2)2[PF] via a number of different synthetic pathways:
- Metathesis reactions with other salts
- Reactions with strong Lewis acids
- Reactions with Brønsted acids
- Halide abstraction
- Hydride and Alkyl abstraction
- Addition or coordination to the cation of a WCA salt
- Direct oxidation of a substrate

