Synthesis and Reactions of a Lewis Acid
The Phosphocenium-Salts
Proofing the classification as a Lewis Superacid we showed that PhF→Al(ORF)3 abstracts fluoride also from unusual sources like [SbF6]–.[1] Now we were interested to test, if this Lewis acid abstracts as well chloride from Cp*ECl2 and (Cp*)2ECl. The new pnictocenium salts [Cp*PCl]+[μCl]– (1a), [Cp*PCl]+[ClAl(ORF)3]– (1b), [Cp*AsCl]+[ClAl(ORF)3]– (2, figure 2), and [(Cp*)2P]+[μCl]– (figure 3) with μCl = (FRO)3Al-Cl-Al(ORF)3 and ORF = OC(CF3)3 were prepared by halide abstraction from the respective halopnictines with the Lewis superacid PhF→Al(ORF)3.[1,2]
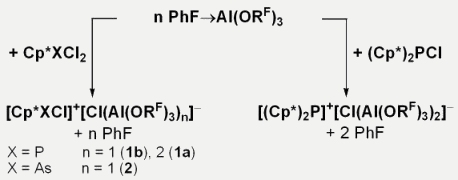
In all X-ray crystal structures of the here reported half- or sandwich cations the Cp* rings are attached in an η2-fashion (figures 2a and 3a). The two new weakly coordinating anions (WCAs) [μCl]– and [ClAl(ORF)3]– are formed by using one or two equivalents of the Lewis superacid PhF→Al(ORF)3. These WCAs stabilize the highly reactive cations even in solution (PhF or 1,2-F2C6H4) at room temperature.
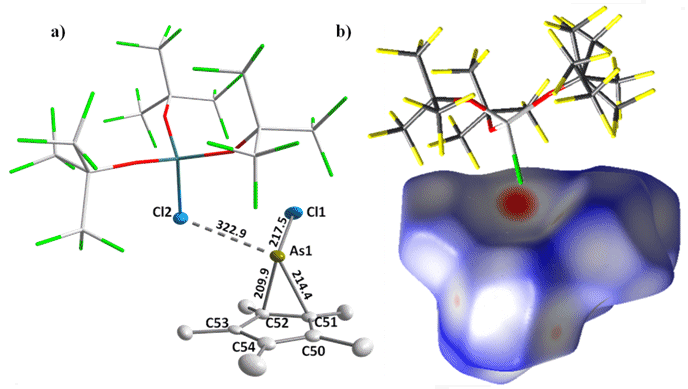
Figure 2. a) Solid-state structure of [Cp*AsCl]+[ClAl(ORF)3]– (2). Thermal ellipsoids of the cation are shown at the 50% probability level. The cationic structure of the phophocenium species is analogous, except for the absence of a stronger cation-chlorine contact. b) Hirshfeld surface of the cation of 2 showing the delocalized nature of the positive charge.
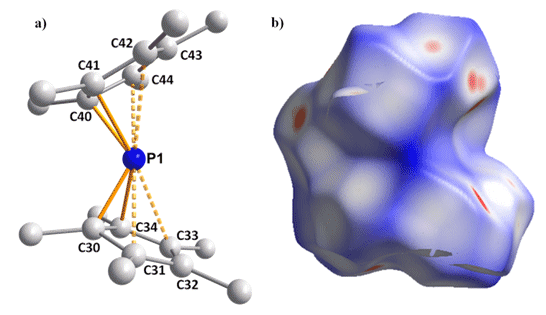
Figure 3. a) Cationic section of the solid-state structure of [(Cp*)2P]+. b) Delocalized Hirshfeld surface.
With the Lewis Superacid we were able to generate the pnictocenium cations [Cp*ECl]+ (E = P, As) and [(Cp*)2P]+ as so far unknown η2-bound Cp* complexes of the main-group elements P and As by halide abstraction. With [(Cp*)2P]+ we openly report on the last missing bissandwich cation of the heavier pentele elements. The Synthesis of 1a and 2 opens the new field of the halo-substituted pnictocenium ions [Cp*EX]+.
The experimentals nicely agree with the reported CIA values, proving the high potential of the Lewis superacid PhF→Al(ORF)3 to form [ClAl(ORF)3]– or [μCl]– as very weakly coordinating novel counterions. The strong Lewis acidity and the non-oxidizing character of PhF→Al(ORF)3 suggest to employ the superacid in processes, where highly reactive, but low valent cations have to be stabilized.[2]
Literature
[1] Lutz O. Müller, Daniel Himmel, Julia Stauffer, Gunther Steinfeld, John Slattery, Gustavo Santiso-Quiñones, Volker Brecht, Ingo Krossing* (2008): „Simple Access to the Non-Oxidizing Lewis Superacid Ph F→Al(ORF)3 (RF = C(CF3)3).”, Angew. Chem. 120, 7772-7776.
[2] Anne Kraft, Jennifer Beck, Ingo Krossing: „Facile Access to the Pnictocenium Ions [Cp*ECl]+ (E = P, As) and [Cp*2P]+. Chloride Ion Affinity of Al(ORF)3.“ Chem. Eur. J. In Press

