Direct Fluorination
Direct Fluorination
In cooperation with the local Department of Microsystems Engineering (IMTEK) mini (≈1mm) and micro (<1mm) structured reactors for the direct fluorination are being developed.
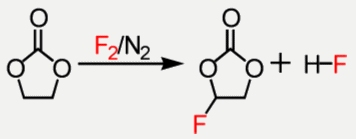
The direct fluorination using molecular fluorine is a very exothermic reaction. The formation of a F-C and a H-F bond out of a F-F and a C-H bond is an exothermic reaction with a reaction enthalpy of around -430 kJ/mol. The energy of a C-C bond is around 360 kJ/mol. This means that the appearing energy of a single fluorination is, in theory, enough to crack the carbon backbone of the substrate. To minimize this effect a strong temperature control is necessary. The very small channels of our reactors have a large surface to volume ratio and because of this it is possible to discharge the heat into the reactor almost instantly. Additionally this method is by far safer than working with bath processes.
The main reaction targets for the fluorination experiments are the perfluorination of alcohols and the selective fluorination of cyclic carbonates. The fluorinated alcohols are one of the basic building blocks for the weakly coordinating anions. The cyclic carbonates like the ethylene carbonate are already used solvents for lithium ion battery technologies. The partially fluorination improves their stability.