Physical Data of ILs, Part 7
Table 14: Experimental (taken from the given references) and calculated melting points according to equations (9) and (10) (for details see ref 10), as well as the COSMO-RS interaction enthalpies HvdW0 and Hring necessary for the prediction of the melting point of the investigated ILs. All energies are given in kJ mol–1.[10]
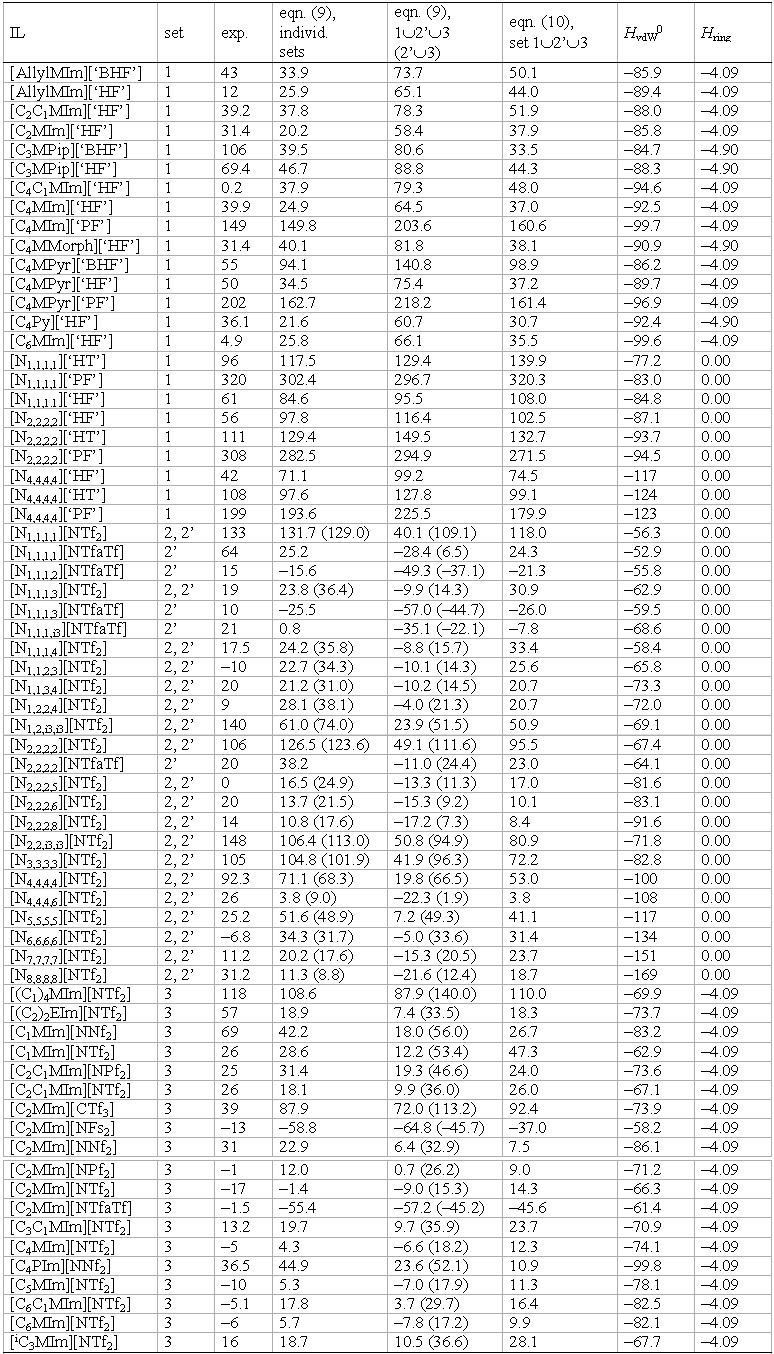
Set 1 consists of a series of 24 salts with weakly coordinating aluminate [Al(ORF)4]– (RF = C(H)(CF3)2 ([‘HF’]–), C(CH3)(CF3)2 ([‘HT’]–), C(CF3)3 ([‘PF’]–)) and borate [B(ORF)4]– (RF = OC(H)(CF3)2, [‘BHF’]–) counterions.
Set 2 consists of a series of salts with ammonium cations.
Set 3 consists of a series of monocyclic imidazolium compounds with perfluorinated anions related to [NTf2]–.
[10] U. Preiss, S. Bulut, I. Krossing, J. Phys. Chem. B 2010, 114, 11133.

