Physical Data of ILs, Part 2
Physical Data of [Al(hfip)4]−/[B(hfip)4]− and [Tf2N]− ILs ― Own Determinations (continued)
Table 5: Experimentally determined static dielectric constants (εS) of the [Al(hfip)4]– ILs investigated in this study (column 2–3) as extrapolated by the CC (Cole-Cole) and CD (Cole-Davidson) models; all measurements were carried out at 70 °C. Column 4–6: electrical dipole moments (μ) in Debye units (1 D = 3.3x10-30 Cm) with referred to the centre of mass ― from single point DFT calculations on (RI)-BP86/SV(P) level by using crystal structure coordinates of the anion (A–), the cation (C+) and the ion pair (C+A–) from asymmetric unit.[12]
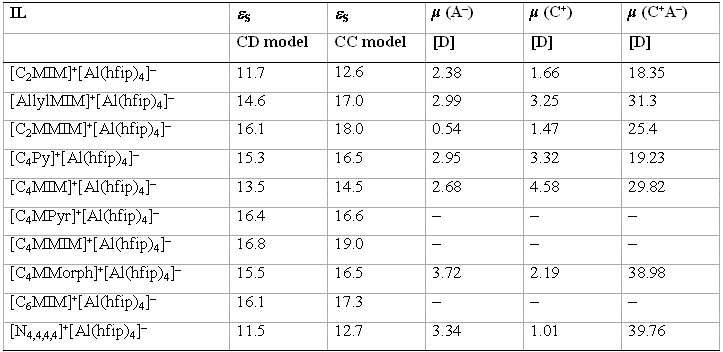
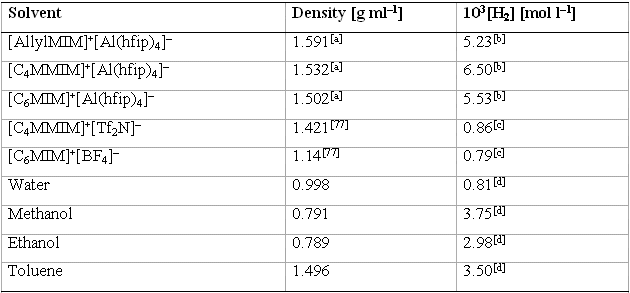
Table 6 : Solubility of H2 in [Al(hfip)4]– ILs and in other solvents previously reported, at 0.101 MPa (1 atm).[12]
[a] Densities were calculated at 298 K by using the previously described method (for details see ref 7). [b] Solubility of H2 at 0.101 MPa (1 atm) and 298 K, calculated from the solubility at 10.1 MPa, with the assumption that it changes linearly with the partial pressure. [c] Solubility of H2 at 0.101 MPa (1 atm) and 298 K. [d]Solubility of H2 at 0.101 MPa (1 atm) and 293 K.
[7] U. Preiss, J. M. Slattery, I. Krossing, Ind. Eng. Chem. Res. 2009, 48, 2290.
[12] S. Bulut, P. Klose, M.-M. Huang, H. Weingärtner, P. J. Dyson, G. Laurenczy, C. Friedrich, J. Menz, K. Kümmerer, I. Krossing, Chem. Eur. J. 2010, 16, 13139.
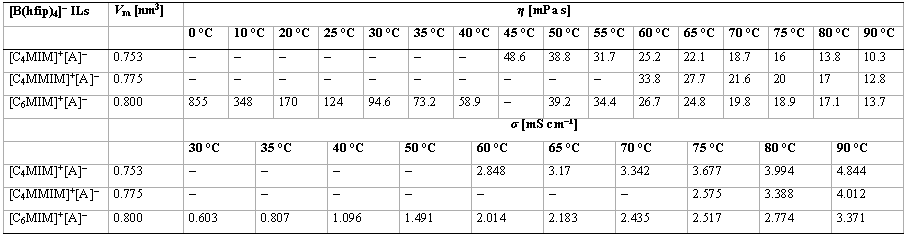
Table 7: Temperature-dependent viscosities (η) and conductivities (σ) of the [B(hfip)4]– (= [A]–) ILs and their molecular volumes (Vm).[18]
[18] S. Bulut, P. Klose, I. Krossing, Dalton Trans. 2011, 40, 8114