Nonclassical CO complexes of coinage metals with WCAs
The two homologues series of the coinage metal carbonyl cations [Cu(CO)n]+ and [Ag(CO)n]+ (n = 1-4) have been studied intensively. Each of the eight complexes has been synthesized in the gasphase and subsequently the bondenergies of the carbonyl ligands to the metal cation were determined.[1]
Today only a few homoleptic gold carbonyl complexes are known. This is in disagreement with the CO-bondenergies in the [Au(CO)2]+-complex, which is so stable, that even under vacuum none of the ligands are abstracted.[2]
The copper carbonyl complexes [Cu(CO)(CH2Cl2)3][PF], [Cu(CO)][HT], [Cu(CO)2][HF] and [Cu(CO)3][ClAl(OC(CF3)3)3] were synthesized by our group. These compounds are characterized with spectroscopic methods as well as single-crystal X-ray analysis. Furthermore we were able to synthesize the silver monocarbonyl complex [Ag(CO)][HT], the silver bis-carbonyl complex [Ag(CO)2][HF] and the gold carbonyl complex [Au2(CO)2Cl][PF].
[PF] = [Al(OC(CF3)3)4]-, [HF] =[Al(OC(H)(CF3)2)4]-, [HT] = [Al(OC(CH3)(CF3)3)4]-
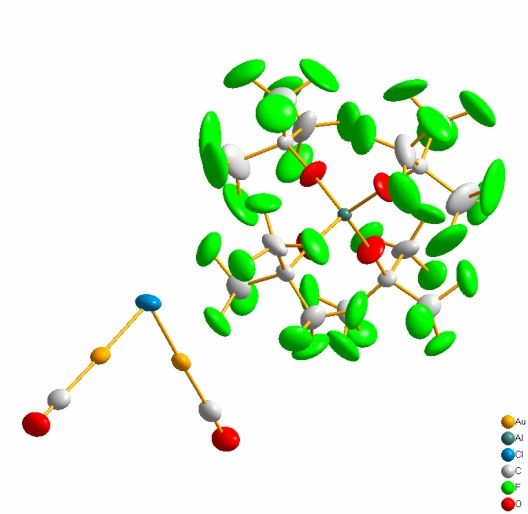
[Au2(CO)2Cl][Al(OC(CF3)3)4]
[1] F. Meyer, Y.-M.Chen, P. B. Armentrout, J. Am. Chem. Soc. 1995, 117, 4071.
[2] A. J. Lupinetti, V. Jonas, W. Thiel, S. H. Strauss, G. Frenking, Chem. Eur. J. 1999, 5, 2573.

