Lewis Superacidity?
The definition may be seen in analogy to Brønsted acids: Brønsted superacids are stronger than the strongest conventional Brønsted acid, 100% H2SO4.
"Molecular Lewis acids, which are stronger than monomeric SbF5 in the gas phase, are Lewis Superacids."
We propose using the FIA as a quantitative measure for Lewis acidity (Table 1). So Lewis acids with a FIA higher than that of monomeric SbF5 (489 kJ/mol) are termed Lewis superacids.[2]
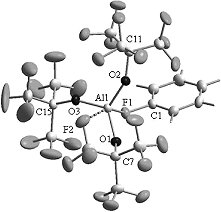
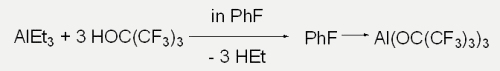
Because of the tendency of monomeric AlBr3 and AlI3 towards aggregation the halogenides were substituted for the bulky perfluorinated alkoxy ligand ORF (ORF = OC(CF3)3). According to quantum chemical calculations the FIA of Al(ORF)3 is 537 kJ/mol (Table 1). The isolation of the new superacid Al(ORF)3 from different solvents under ambient conditions was very difficult, because of self-decomposition with C-F activation. After having changed the solvent to fluorobenzene the adduct PhF→Al(ORF)3 was formed in 98% yield and can be easiliy handled as a room temperature sensitive powder.
Literature
[2] Lutz O. Müller, Daniel Himmel, Julia Stauffer, Gunther Steinfeld, John Slattery, Gustavo Santiso-Quiñones, Volker Brecht, Ingo Krossing* (2008): „Simple Access to the Non-Oxidizing Lewis Superacid Ph F→Al(ORF)3 (RF = C(CF3)3).”, Angew. Chem. 120, 7772-7776.