Fluorid Ion Affinity (FIA)
Since the pioneering work of N. Bartlett et al.[1] it is known that the fluoride ion affinity (FIA) is a reliable measure of the Lewis acidity, combining the strength of a Lewis acid A(g) with the energy that is released upon binding a fluoride ion F¯:
![]()
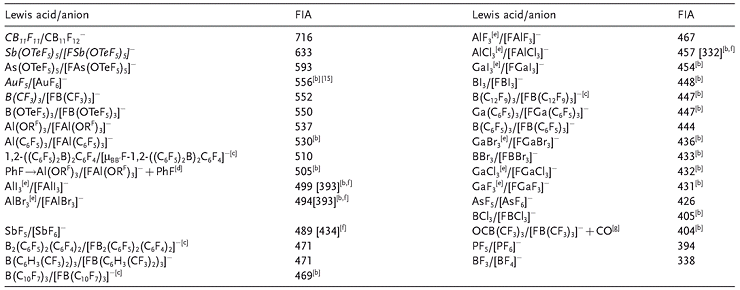
The higher the FIA of the parent Lewis acid A of a given WCA, the more stable it is towards decomposition on thermodynamic grounds. The simplest and most general access to reliable FIA values now comprises the use of quantum chemical calculations in isodesmic reactions. Table 1 shows the calculated FIAs of a representative set of strong neutral Lewis acids.
Table 1: Representative overview of known Lewis acids and their corresponding fluoride complexes [a] [14]
[a] Unstable and hitherto unknown lewis acids that have only been determined theoretically are shown in italics (stability refers to standard conditions: 298 K, 1013 mbar). The empty row marks the border between normal and Lewis superacids. If not otherwise stated, FIA values [kJ mol-1] are taken from Ref. [6a] or were calculated as part of this work using the same methodology as in [6a]. [b] This work. [d] RF=C(CF3)3. [e] Monomeric EX3, (E=Al, Ga). [f] Values in brackets are with respect to the standard state of the Lewis acid, that is, solid for AlX3,[16] and liquid for SbF3.[17] We are aware that for higher aggregates such as SbnF5n, and AlnX3n the calculated FIA values reach much higher numbers, but the gas-phase value of 489 kJ mol-1 gives a reasonable approximation of the average Lewis acidity of monomers and oligomers present in the condensed or liquid phase. [g] OCB(CF3)3 reacts with F- to give [F(O)CB(CF3)3]- .[18]
Literature
[1] T. E. Mallouk, G. L. Rosenthal, G. Mueller, R. Brusasco, N.Bartlett, Inorg. Chem. 1984, 23, 3167 – 3173.
[2] Lutz O. Müller, Daniel Himmel, Julia Stauffer, Gunther Steinfeld, John Slattery, Gustavo Santiso-Quiñones, Volker Brecht, Ingo Krossing* (2008): „Simple Access to the Non-Oxidizing Lewis Superacid Ph F→Al(ORF)3 (RF = C(CF3)3).”, Angew. Chem. 120, 7772-7776.
[4] Anne Kraft, Jennifer Beck, Ingo Krossing: „Facile Access to the Pnictocenium Ions [Cp*ECl]+ (E = P, As) and [Cp*2P]+. Chloride Ion Affinity of Al(ORF)3.“ Chem. Eur. J. In Press
[14] According to our definition, Me3Si[C6F5CTf2](Tf =triflate), [10a] which reacts with F to give Me3SiF and [C6F5CTf2], is not a Lewis superacid; its stable enol form (SiO bond) has a FIA of only 407 kJ/mol.
[15] T he FIA has been previously calculated with a differentmethod. [20b] In contrast to those results, the FIAvalues presented herein were calculated at the BP86/SV(P) level of theory to allow comparison.
[16] The procedure for the determination of ΔHsolid for AlX3 is given in the Supporting Information of Müller L. O., Himmel D., Stauffer J., Steinfeld G., Slattery J. M., Santiso-Quinones G., Brecht V., Krossing I.: Simple access to the non-oxidizing Lewis superacid PhF->Al(ORF)3 (RF = C(CF3)3). Angew Chem Int Edit, 2008; 47: 7659-7663
[17] H. D. B. Jenkins, I. Krossing, J. Passmore, I. Raabe, J. Fluorine Chem. 2004, 125, 1585 – 1592.
[18] M. Finze, E. Bernhardt, H. Willner, C.W. Lehmann, Angew. Chem. 2003, 115, 1082 – 1085; Angew. Chem. Int. Ed. 2003, 42, 1052 – 1055.
Group Publications
Lutz O. Müller, Rosario Scopelitti, Ingo Krossing (2008): “The Fluorinated Lithiumalkoxide LiORF (RF = C(CF3)2Mes), the Alcohol RFOH and Attempts to use them as Precursors for New WCAs.”, Z. Allg. Anorg. Chem. 634, 1035-1040.
I. Krossing*, I. Raabe, “Non coordinating anions: fact or fiction? A survey of likely candidates.” Angew. Chem. 2004, 116, 2116-2142.