Chloride Ion Affinity (CIA)
Typically the strength of a strong Lewis acid, is classified by its – usually calculated – FIA value (FIA = fluoride ion affinity).[1] In analogy to the FIA, the chloride ion affinity CIA of any Lewis acid A
![]()
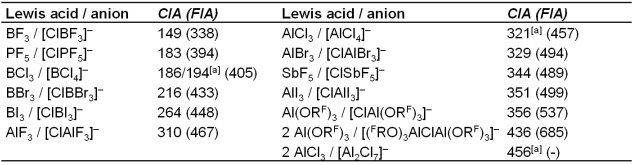
allows for a quantitative ordering of the Lewis acidity with respect to chloride as a base (Table 1). In comparison to the FIA, the CIA values are lower but (almost) the same ion affinity trends were observed. The CIA of Al(ORF)3 is with 356 (giving [ClAl(ORF)3]–) and 436 kJ mol–1 (giving [μCl]– = [(FRO)3AlClAl(ORF)3]–) in the typical range of very strong Lewis acids and also exhibits a higher chloride ion affinity than SbF5. Thus, also with respect to gaseous SbF5, Al(ORF)3 and AlBr3 a Lewis Superacid.[2]
Table 1: Overview on the calculated CIA-values of classical Lewis acids and Al(ORF)3. The FIA-values of the acids, with respect to the homologous fluoride complexes,[1] are included in parentheses. CIA (FIA) values are given in kJ mol–1.
[a] Reference system at G3MP2 level at 298 K.
Literature
[1] Lutz O. Müller, Daniel Himmel, Julia Stauffer, Gunther Steinfeld, John Slattery, Gustavo Santiso-Quiñones, Volker Brecht, Ingo Krossing* (2008): „Simple Access to the Non-Oxidizing Lewis Superacid Ph F→Al(ORF)3 (RF = C(CF3)3).”, Angew. Chem. 120, 7772-7776.
[2] Anne Kraft, Jennifer Beck, Ingo Krossing: „Facile Access to the Pnictocenium Ions [Cp*ECl]+ (E = P, As) and [Cp*2P]+. Chloride Ion Affinity of Al(ORF)3.“ Chem. Eur. J. In press.

