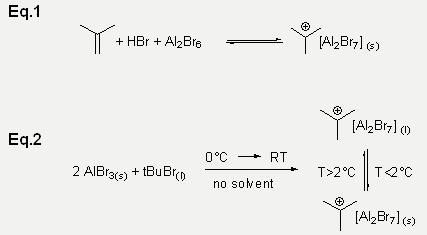
Reactions with Aluminium bromide
AlBr3 with a FIA of 494 kJ/mol is known to be a Lewis Superacid. We were interested in testing the the superacidic behaviour of AlBr3. Lewis/Brønsted superacid media such as “Magic Acid” (HFSO3/SbF5) having H0 in the range –17 to –27 are used to stabilize tert-butyl cation.[1] The superacidic properties of AlBr3 apparent strong enough to shift the equilibrium of Equation 2 almost completely to the right hand side. If this were not the case deprotonation of the alkyl cation to alkene and subsequent decomposition by cationic oligomerization would occur rapidly. Finally the reactions of tert-butyl bromide and AlBr3 were carried out without solvent. Tert- Butyl bromide was dissolved in alumiunium bromide. Upon reaction at 0°C (15 min) and subsequent warming to RT a liquid with high density and a viscosity like olive oil formed with stirring. The solution turned out to be remarkably stable. At 2°C the oil reversibly crystallizes characterizing the compound as a room temperature ionic liquid (Eq.2). X-ray analysis confirm the compound as C(CH3)3+[Al2Br7]–.

Characterisation:
NMR: The presence of the tert-butyl cation was proven as the main product (δ1H = 2.6, δ13C = 325 (Cq), 49 (CH3), δ27Al = 82.4) besides some weak signals, which suggest the formation of isobutene oligomers. Also IR and Raman confirm the composition.
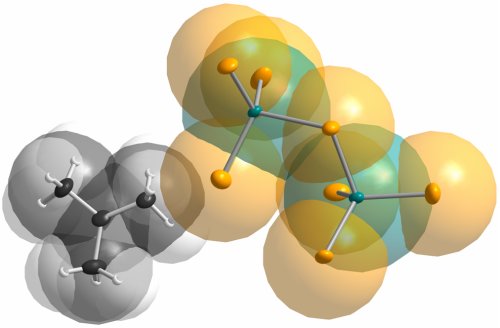
Fig. 4 Molecular structure of C(CH3)3+[Al2Br7]–. Thermal ellipsoids are shown at the 50% probability level. Selected bond length [Ǻ] and angles [°]: Br11-Al1 2.222(2), Br13-Al1 2.246(2), Br12-Al1 2.252(2), Br14-Al1 2.435(2), Br14-Al2 2.406(2), Br21-Al2 2.257(2), Br22-Al2 2.264(2), Br23-Al2 2.254(2); Al1-Br14-Al2 108.03(7), Br11-Al1-Br12 114.26(9), Br11-Al1-Br13 112.89(9), Br12-Al1-Br13 114.22(9), Br21-Al2-Br22 113.03(9), Br22-Al2-Br23 113.02(9), Br21-Al2-Br23 113.79(9), C11-C10-C13 120.2(7), C12-C10-C13 120.7(7), C11-C10-C12 119.1(7).
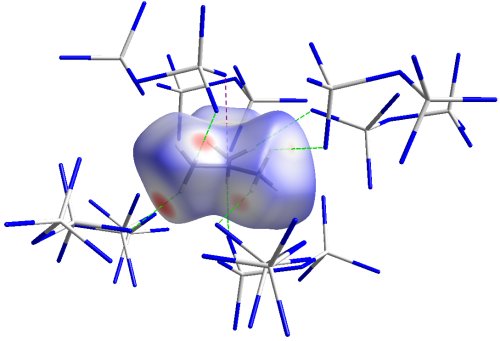
Fig. 5 Hirshfeld surface of the C(CH3)3+ cation with surrounding counterions. Red colour indicates contact distances below, blue colour above sum of van der Waals radii.
In a closed flask or NMR tube slow partial decomposition occurs at RT with formation of (mainly) protonated isobutene oligomers until an HBr equilibrium pressure is reached. This can be largely prevented by adding an HBr pressure of ~0.3 bar.
We have shown that this novel tertiary carbocation salt can be easily synthesized and handled at room temperature. It opens the way to be used as a readily available source for the tert-butyl cation. Thus, we assume that this straight forward and also in larger sale available superacidic IL represents a starting material that will be widely used for further reactions and investigations.
Literature
[1] G. A. Olah, G. K. S. Prakash, Á. Molnár and J. Sommer, Superacid Chemistry, John Wiley & Sons, Inc., New Jersey, 2009.