Weakly Coordinating Anions
 | A Brief Introduction to Weakly Coordinating Anions Article on Weakly Coordination Anions in "Chemie Plus" here (pdf, 200 kB) Brief introduction to the chemistry of WCA's see here |
A Brief Introduction to Weakly Coordinating Anions
The prime role of WCAs is to suppress strong cation-anion interactions and to replace the few strong electrostatic interactions in classical salts by a multitude of weak interactions. To achieve this goal, the majority of more recent WCAs are rather large and exhibit diameters in the nanometer range; moreover their “surfaces” are often covered by poorly polarizable fluorine atoms that produce a “Teflon”-type coating additionally dampening the efficiency of ion pairing and increasing the (kinetic) inertness and (thermodynamic) stability of these anions against ligand coordination or abstraction as well as oxidation. Overall this leads to improved solubilities of WCA-salts in less or even non polar solvents, reduces ion pairing in these solvents and ultimately also leads to electric conductivity in these media. Figure 1 shows a personally selected overview on currently used WCAs.
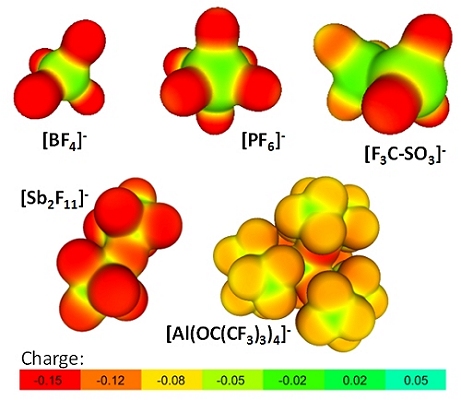
Figure 1: Red coloration indicates high accumulation of negative charge
Fluorinated Alkoxyaluminates as WCAs: A Comparison to Competitors
Among the above mentioned WCAs, the fluorinated alkoxyaluminates [Al(ORF)4]– are a newer addition that were first published by S. Strauss in 1996 and were complemented by our group since 1999. Currently we are aware of at least 30 groups worldwide that use the favorable properties of this chemically robust and easily in large scale or even commercially at www.iolitec.de available WCA class.
Article on WCAs in "Chemie Plus" 2011 (pdf, 200 kB)


