Fluorinated Alkoxyaluminates
Fluorinated Alkoxyaluminates as WCAs: A Comparison to Competitors
Among the above mentioned WCAs, the fluorinated alkoxyaluminates [Al(ORF)4]– are a newer addition that were first published by S. Strauss in 1996 and were complemented by our group since 1999. Currently we are aware of at least 30 groups worldwide that use the favorable properties of this chemically robust and easily in large scale or even commercially at www.iolitec.de available WCA class.
With Respect to Coordinating Ability: Since the major purpose of a WCA is to reduce the overall cation-anion interaction, the performance of the coordination ability of the [Al(ORF)4]– aluminates in comparison to other well known WCAs may best be visualized by from a projection of the PBE0/TZVPP calculated electrostatic potential onto a 0.005 e– Bohr–3 isodensity surface of the aluminate WCA in comparison to classical WCAs (Figure 2; red coloration indicates high accumulation of negative charge).
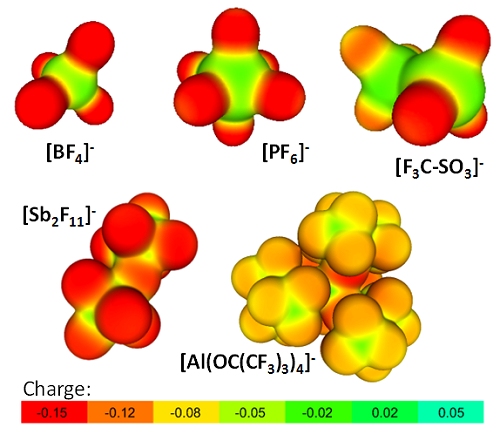
Figure 2. Projection of the PBE0/TZVPP calculated electrostatic potential onto a 0.005 e– Bohr–3 isodensity surface of [Al(OC(CF3)3)4]– in comparison to classical WCAs.
When comparing with the often employed fluorinated tetraarylborates (Figure 3), one also realizes the favorable performance of the aluminates. From this projection also follows that the weak coordination ability of the [Al(OC(CF3)3)4]– aluminate is a result of two effects: electronic deactivation of the most basic oxygen alkoxide atoms by the fluorination (see low pKa value of HO-C(CF3)3 of 5.4) and the steric hindrance introduced by the umbrella effect of the bulky C(CF3)3 group preventing electrophiles/cations to attack these most basic sites.
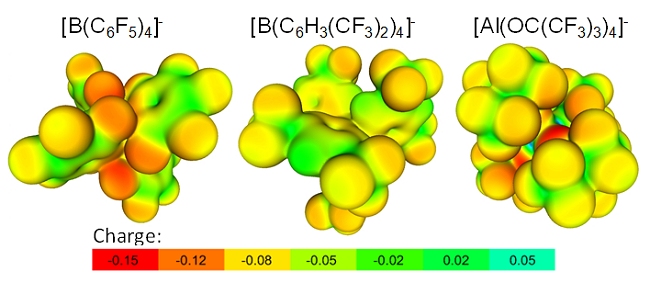
Figure 3. Projection of the BP86/SVP calculated electrostatic potential onto a 0.005 e– Bohr–3 isodensity surface of [Al(OC(CF3)3)4]– in comparison to fluorinated tetraarylborates.
The final Figure 4 of this series, compares the [Al(ORF)4]– aluminates with the difficult to synthesize, but chemically extraordinary robust carborane based WCAs. One realizes from this Figure 4, that only the most advanced trifluoromethylated carborane WCAs reach the potential of the [Al(ORF)4]– aluminates in terms of for coordination available negatively charged sites. The more common only partially chlorinated carborane based anions exhibit higher and accessible surface charges. In agreement with this, these carborane ions tend to get easily coordinated, but can sustain extreme electrophilicity in such a coordinated state (e.g., they tolerate a coordinated AlMe2+ cation).
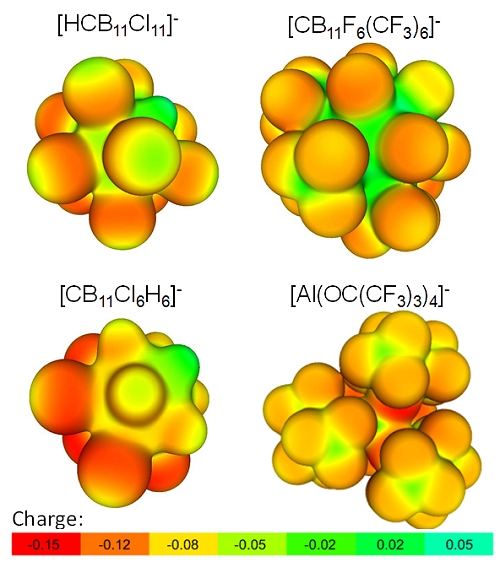
Figure 4. The [Al(ORF)4]– aluminates in comparison with the difficult to synthesize, but chemically extraordinary robust carborane based WCAs.

