Cationic Brønsted acids
The stabilisation of cationic brønsted acids requires weakly coordinating ions (WCAs) of low basicity. Some years ago, an easy synthesis route to multigram amounts the protonated diethylether salt H(OEt)2)2+[Al(OC(CF3)3)4]- was developed in our workgroup.

Dissolved in low polar solvents like toluene or dichloromethane, this salt is an extremely efficient initiator for the cationic isobutene polymerisation even in micromole amounts!
Generally, by reducing the polarity of the solvent, the acidity of cationic Brønsted acids is drastically increased compared to neutral ones. Except our “traditional” alkoxyaluminate anions, we also use halogenoaluminates AlX4- and Al2X7- (X = Cl, Br, I) for stabilising cationic Brønsted acids like protonated mesitylene.
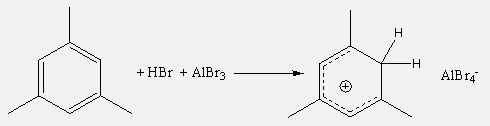
Very recently, we managed to synthesise CMe3+[Al2Br7]. Note that the tert-butyl cation is nothing but protonated isobutene. Remarkably, this compound is a room-temperature ionic liquid with a melting point of 2°C!
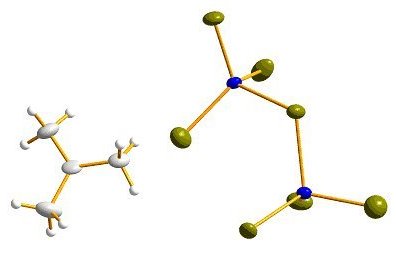
Crystal structure of [C(CH3)3][Al2Br7]. Thermal ellipsoids are shown at the 50% probability level.

Liquid [C(CH3)3][Al2Br7] at RT, right: solid [C(CH3)3][Al2Br7] at 0°C

