Protoned ether as initiator
[H(OR2)2][Al(ORF)4] - a promising initiator for the polymerisation of isobutylene
Choosing the fundamental approach, Krossing et al. were able to synthesize novel proton-based initiators for the polymerisation of isobutylene.[92] These thermally stable salts of protonated dialkylethers, like [H(OR2)2][Al(ORF)4] (R = Et2; iPr2 and RF = C(CF3)3) are strong cationic Brønsted acids and therefore a promising species to initiate the polymerisation of isobutylene.[3-6] The weakly coordinating nature of the counterion [Al(ORF)4]– is of great importance, as it enables a living cationic polymerisation. This fact makes an addition of extra additives, in order to obtain well defined PIB obsolete.[93-95]
Quantum chemical calculations in the gas phase show that the initiation of the polymerisation is thermodynamically disfavoured (Scheme 3).
![]()
Scheme 3 : Thermodynamic evaluation of the formation of the tert-butyl cation (MP2/def2-QZVPP); the initiating step of the polymerisation of isobutylene.
Therefore one has to ask: Why is the oxonium acid [H(OR2)2]+ such a good initiator for the polymerisation of isobutylene? A satisfying answer gives the unified pH scale for all phases of Himmel et al.[96, 97] This unified pH scale takes the interaction with solvent molecules in account and more importantly allows comparison of Brønsted acidities of [H(OR2)2]+ in different media, e.g. CH2Cl2 and Et2O. A calculated Born-Haber-Fajans cycle reveals the importance of considering such solvent effects (Scheme 4).
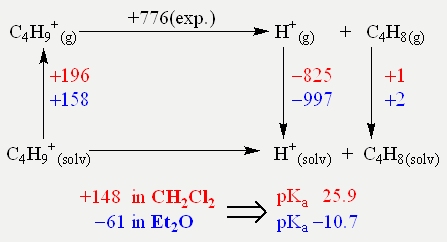
Scheme 4 : Born-Haber-Fajans cycle showing the importance of solvent effects, when it comes to acidities of [C4H9]+. Energie values are given in kJ mol–1.
Thus the poylmerisation only takes place in CH2Cl2 as the pKa value of the primarily formed tert-butyl cation [C4H9]+ is 16 magnitudes higher than in Et2O. In other words: The degree of protonation of isobutylene in CH2Cl2 is higher than in Et2O because [H(OEt2)2]+ is a stronger acid in CH2Cl2 than in Et2O. This theoretical approach was backed by a number of experiments. Thus adding 50 μl of Et2O to the reaction mixture of [H(OEt2)2][Al(ORF)4] in CH2Cl2 no polymerisation of isobutylene could be observed upon the addition of isobutylene.

