Thermodynamic calculations
By combining experimental data, empirical correlations, and quantumchemical calculations, Born-Fajans-Haber cycles (BFHCs) can be constructed to gain a deeper insight into reaction thermodynamics. For example, The formation of the above described CMe3+[Al2Br7] can be reproduced very well in silico. Even the fact that it´s a liquid at room temperature!
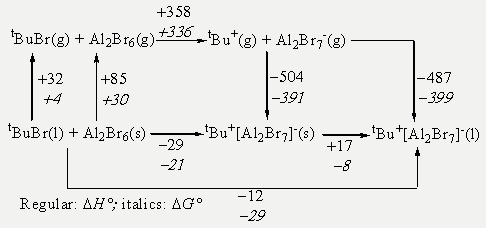
Born-Haber-Cycle to assess the reaction enthalpy (top) and Gibbs reaction enthalpy (down) in kJ/mol: Values in ithalics are standard Gibbs energies, those in regular font are standard enthalpies at 298 K, 1 bar.

