Energy conversion
 | Carbon dioxide as greenhouse gas Hydrogenation of carbon dioxide to methanol Phase diagram of methanol synthesis from CO2 und H2 Investigations of heterometallic catalyst systems Use of Ionic liquids (ILs) in heterogeneous catalyst systems |
The role of carbon dioxide as greenhouse gas and its contribution to global warming are widely discussed in recent years. Carbon dioxide is produced in many industrial processes, where fossil fuels or biomass are combusted. The interest in lowering CO2 emission and its use as carbon source has significantly increased in last decades. One attractive approach is the conversion of CO2 to methanol, due to the high importance of methanol as energy storage material, fuel and precursor for useful organic chemicals.
The hydrogenation of CO2 to methanol can be described by the following equation:
![]()
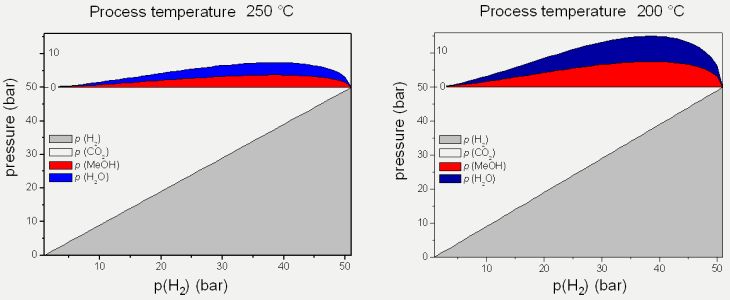
The synthesis of methanol is exotherm. During the reaction a decrease in the number of moles occurs. According to Le Chatelier`s principle, maximum conversion is obtained at low temperature and high pressure. Today industrial production of methanol is performed at high temperatures (250 °C) and pressure (50 bar) with Cu-ZnO-based mixed systems as catalysts. Lowering of the reaction temperature from 250 °C to 200 °C would lead to a doubling of the yield of methanol (Figure 1).
Figure 1: Phase diagram of methanol synthesis from CO2 und H2 at 250 °C (left) und 200 °C (right).
To increase the efficiency of CO2 conversion by lowering the reaction temperature, optimization of the current catalysts is inevitable. In this project we investigate novel syntheses of heterometallic Cu/ZnO/ZrO2 and Cu/ZnO/ZrO2(Al2O3) catalyst systems and their fluoride/fluoroxide analogs with different physical (morphology, specific surface area and particle size distribution) and catalytic properties. The catalysts are synthesized via hydrolysis-condensation route from the corresponding metal salts and/or alcoxides. Cu/ZnO/ZrO2 and Cu/ZnO/ZrO2(Al2O3) catalysts with varying compositions using different salts as educts and reaction parameters (solvent, precipitant, temperature, pH, ageing time) have been prepared, and their catalytic properties at different reaction temperatures and pressures were determined.
The catalyst systems prepared from metal salts via the co-precipitation route were characterized by TEM, SEM/EDX, XRD and BET (Figure 2).
Figure 2: a) and c) TEM images, b) EDX spectrum, d) BET adsorption-desorption isotherm and e) XRD of a nanopartical metal oxide catalyst (Cu/ZnO/ZrO2); f) AP MiniPlant testing system with a tubular reactor for catalyst tests.
To investigate the activity and selectivity of the synthesized catalysts an AP MiniPlant system has been commissioned. During the CO2-hydrogenation the ratio CO2/CH3OH as well as the amount of by-products can be monitored.
Another approach is the use of Ionic liquids (ILs) in heterogeneous catalytic transformation of CO2 to methanol. ILs could not only act as a solvent for apolar educts (CO2 and H2), but also play an important role in promoting the reactivity of catalytic active sites. Finally the polar products (CH3OH and H2O) could be removed due to their low affinity to ILs.